0727
Multinuclear MR and PET for Studying Energetic Adaptations in Individuals at Increased Risk for Alzheimer’s Disease1Department of Radiology, NYU School of Medicine, New York, NY, United States, 2Department of Neurology, Weill Cornell Medical College, New York, NY, United States, 3Department of Psychiatry, NYU School of Medicine, New York, NY, United States
Synopsis
31P-MRS directly assesses metabolites linked to cellular metabolism, which may be altered at the early stages of Alzheimer’s disease (AD). A major challenge for 31P-MRS is the low sensitivity of the 31P nucleus. Therefore, 31P-MRS has only been used sporadically in AD research. To address this, we built a highly-sensitive dual-nuclei (31P/1H) radio frequency coil array and acquired whole-brain 31P-MRS data from cognitive normal subjects at increased risk for AD, who had previously received FDG and amyloid-PET evaluations. Our goal was to detect energetic abnormalities in this pre-clinical population and compare 31P-MRS findings with established PET-based biomarkers of AD.
Introduction
It has been postulated that energy metabolism dysregulation could play an important role in the early pathogenesis of AD.1,2 Gaining insight into the early stages of the disease would allow for the validation of candidate disease-modifying treatments. Current studies of metabolic impairment in AD mostly rely on the assessment of glucose uptake using [18F]-fluorodeoxyglucose (FDG) positron emission tomography (PET),3 while amyloid burden, a hallmark of AD, can be assessed using amyloid PET.4 These studies would greatly benefit from 31P-MRS, which measures key metabolic molecules such as nucleotide triphosphate (NTP), which is mainly composed of adenosine triphosphate (ATP), and phosphocreatine (PCr). Other detectable metabolites include phosphomonoesters (PME), phosphodiesters (PDE), and inorganic phosphate (Pi). Therefore, 31P-MRS allows for a direct assessment of metabolites linked to cellular energy metabolism and energetics complementing existing PET-based imaging markers. Despite its potential usefulness, 31P-MRS has been challenged by the low MR sensitivity associated with the 31P nucleus and the low concentration of 31P containing metabolites in the brain, which has limited its application in AD research.5-7 To address this issue, we developed a highly-sensitive dual-nuclei radiofrequency coil array for whole-brain 31P and 1H-MR imaging and spectroscopy at 3T. Herein, we used the device to acquire whole-brain 31P-MRS data from cognitively normal adults with a first degree family history of AD and/or APOE4 genotype (i.e. genetic risk factors for AD), who had previously undergone FDG and amyloid PET scans.8,9 The goal of this study was to detect bioenergetic abnormalities in a population of individuals at increased risk for AD and compare 31P-MRS findings with previously acquired more established PET imaging biomarkers for AD.Methods
We developed a dual-nuclei radio frequency coil array for efficient 1H-MRI and 31P-MRS that consists of two radially interleaved eight-channel arrays for each nucleus (Fig. 1). The device was designed to minimize PET attenuation by consolidating the coil into a two “layer” degenerate-mode birdcage structure (one transmit/receive 1H layer and one transmit/receive 31P layer), moving MRI interface components outside the PET FOV, and enclosing the device in a stealth polycarbonate shell. The MRI interface was set up to drive each degenerate mode birdcage in the circularly-polarized mode for uniform spin excitation, while signal detection was performed in eight-channel phased-array mode for high SNR. We acquired 3D 31P-CSI data from 10 cognitive normal subjects (age 54.1±6.2, 80% female). All participants had at least one first-degree relative whose AD onset was after age 60. Data were acquired on a whole body 3T scanner (Prisma, Siemens Healthineers, Erlangen, Germany) between June and October 2017. Acquisition parameters for weighted k-space sampled 31P-CSI: repetition time: 2000 ms, flip angle: 55o, field-of-view: 240 x 240 x 240 mm, Matrix Size: 8 x 8 x 8 (interpolated to 16 x 16 x 16), acquisition time: 23 min. Ratios of PCr/ATP, and PME/PDE were quantified using AMARES within the JMRUI package.10 Subjects had previously undergone FDG-PET, Pittsburgh compound B (PiB)-amyloid PET and structural MRI evaluations between 2012 and 2015. MPRAGE images from each session were used to co-register PiB, FDG, and 31P-MRSI datasets.8,9 We compared FDG and PiB standard uptake values ratios (SUVR) with 31P metabolite ratios in an AD-vulnerable meta region11 (ADmask).Results
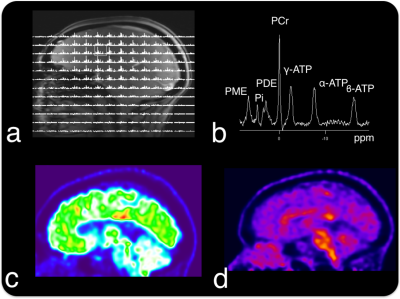
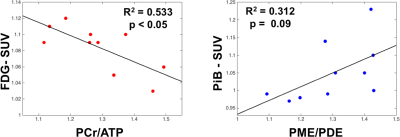
Representative 1H-MRI, 31P-MRS, and PET data are shown in Figure 2. Phosphorus MRS data showed significant associations with PET data that are consistent with previous studies in the early stages of the disease (p <0.05; Fig. 3).12,13Discussion and Conclusion
Preliminary results in cognitively normal subjects at increased risk for AD suggest a connection between cellular energetics (from 31P-MRS) and down regulation of glycolysis (from FDG-PET). In addition, 31P-MRS showed a link between PiB uptake and cell membrane impairment (i.e. increase in PME/PDE). These preliminary results suggest that multinuclear MR-PET provides new insights into changes at the early stage of AD that may allow earlier disease detection and help develop novel targets for treatment. A limitation of this study was that PET and 31P-MRS data were collected in different sessions. Future studies will utilize a simultaneous co-modal 31P-MRS and PET scanner to avoid spurious findings related to physiologic fluctuations (cerebral activation, cogitation, diurnal, circadian, or post-prandial effects) that plague separate PET and 31P-MRS measurements.Acknowledgements
This work was supported by NIH grants 1UL1TR001445 and S10OD021772, and was performed under the rubric of the Center for Advanced Imaging Innovation and Research (CAI2R, www.cai2r.net) at the New York University School of Medicine, which is an NIBIB Biomedical Technology Resource Center (NIH P41 EB017183).
References
1. Gibson GE, Shi Q. A mitocentric view of Alzheimer’s disease suggests multi-faceted treatments. J Alzheimers Dis 2010; 20(0 2):S591.
2. Moreira PI, Carvalho C, Zhu X, Smith MA, Perry G. Mitochondrial dysfunction is a trigger of Alzheimer's disease pathophysiology. 2010; 1802(1):2-10.
3. Herholz K, Salmon E, Perani D, Baron J, Holthoff V, Frölich L, Schönknecht P, Ito K, Mielke R, Kalbe E. Discrimination between Alzheimer dementia and controls by automated analysis of multicenter FDG PET. Neuroimage 2002; 17(1):302-316.
4. Edison P, Archer H, Hinz R, Hammers A, Pavese N, Tai Y, Hotton G, Cutler D, Fox N, Kennedy A. Amyloid, hypometabolism, and cognition in Alzheimer disease An [11C] PIB and [18F] FDG PET study. Neurology 2007; 68(7):501-508.
5. Mandal PK, Akolkar H, Tripathi M. Mapping of hippocampal pH and neurochemicals from in vivo multi-voxel 31P study in healthy normal young male/female, mild cognitive impairment, and Alzheimer's disease. J Alzheimers Dis 2012; 31(s3):S75-S86.
6. Forlenza OV, Wacker P, Nunes PV, Yacubian J, Castro CC, Otaduy MC, Gattaz WF. Reduced phospholipid breakdown in Alzheimer’s brains: a 31P spectroscopy study. Psychopharmacology 2005; 180(2):359-365.
7. Cuénod C-A, Kaplan DB, Michot J-L, Jehenson P, Leroy-Willig A, Forette F, Syrota A, Boiler F. Phospholipid abnormalities in early Alzheimer's disease: in vivo phosphorus 31 magnetic resonance spectroscopy. Arch Neurol 1995; 52(1):89-94.
8. Mosconi L, Tsui W, Murray J, et al. Maternal age affects brain metabolism in adult children of mothers affected by Alzheimer's disease. Neurobiol Aging 2012; 33(3):624. e621-624. e629.
9. Mosconi L, Berti V, Swerdlow RH, Pupi A, Duara R, de Leon M. Maternal transmission of Alzheimer's disease: prodromal metabolic phenotype and the search for genes. Human Genom 2010; 4(3):1.
10. Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson 1997; 129(1):35-43.
11. Landau SM, Harvey D, Madison CM, Koeppe RA, Reiman EM, Foster NL, Weiner MW, Jagust WJ, Initiative AsDN. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging 2011; 32(7):1207-1218.
12. Pettegrew JW, Panchalingam K, Moossy J, Martinez J, Rao G, Boller F. Correlation of phosphorus-31 magnetic resonance spectroscopy and morphologic findings in Alzheimer's disease. Arch Neurol 1988; 45(10):1093.
13. Pettegrew JW, Moossy J, Withers G, McKeag D, Panchalingam K. 31P nuclear magnetic resonance study of the brain in Alzheimer's disease. J Neuropathol Exp Neurol 1988; 47(3):235-248.
Figures