0726
Non-invasive Assessment of Glymphatic Inflow: Measurement of Perivascular Fluid Movement using Diffusion Tensor MRI1Centre for Advanced Biomedical Imaging, University College London, London, United Kingdom, 2Francis Crick Institute, London, United Kingdom, 3Neuroradiological Academic Unit, Department of Brain Repair and Rehabilitation, University College London, London, United Kingdom, 4Leonard Wolfson Experimental Neurology Centre, UCL Institute of Neurology, University College London, London, United Kingdom
Synopsis
The glymphatic system may play a critical role in the parenchymal clearance of amyloid beta, a leading molecular candidate to initiate Alzheimer’s disease. Clinical investigation, however, is currently hindered by an absence of non-invasive techniques for assessment. The movement of fluid in the perivascular space represents a central component of the glymphatic pathway. Here, we present the first non-invasive evaluation of glymphatic function by using an ultra-long echo-time, low b-value, diffusion-tensor sequence targeted to the perivascular space of the rat brain. We demonstrate that this novel technique is sensitive to the fluid movement along perivascular channels that drives glymphatic inflow.
Introduction
The recent identification of the glymphatic system and the dural lymphatic network provide exciting new perspectives on waste clearance mechanisms within the CNS1,2. Concordant observations, using invasive measurements, have been made in the rodent, primate and human brain, suggesting preservation of these pathways across species3,4.
Accumulation of amyloid beta (Aβ) in late onset, sporadic Alzheimer’s disease (AD) patients occurs not because of increased production but due to decreased rates of Aβ clearance5; impairment to the glymphatic system has been shown to markedly reduce Aβ clearance in the mouse brain2,6. Together, this raises the exciting prospect that glymphatic clearance represents a breakthrough target for early detection and therapeutic invention in AD.
Currently, however, the study and clinical assessment of glymphatic clearance is hindered by an absence of non-invasive techniques for assessment. Here we introduce the first non-invasive technique to assess the glymphatic pathway. We apply ultra-long TE, diffusion-weighted MRI sequences to assess fluid movement within perivascular channels surrounding the middle cerebral artery (MCA) of the healthy rat brain (which form essential routes of glymphatic inflow). By using an ultra-long TE, the sequences are designed to null the signal from surrounding tissue and blood to minimise partial volume effects when extracting MRI signals that derive from fluid in the perivascular space. This represents the first non-invasive biomarker of perivascular function, working towards new translational techniques to assess brain clearance pathways and their role in neurodegenerative disease.
Methods
16 anaesthetised healthy male Sprague Dawley rats were scanned at 9.4T (Agilent; 1.75% isoflurane in 0.4L/min medical air and 0.1L/min O2).
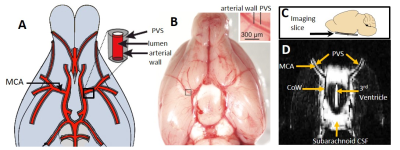
Multiple Direction Diffusion-Weighted imaging (n=10): An axial slice was positioned at the level of the Circle of Willis (see Figure 1). The right perivascular tracts (surrounding the MCA in the axial slice) were aligned with the orientation of the frequency encoding (FE) gradients; the left perivascular tracts then become approximately aligned with the phase encoding (PE) gradients (see Figure 2). A fast-spin-echo diffusion weighted sequence was acquired with the following sequence parameters: TR = 5s, effective TE = 142 ms, 200µm resolution, slice thickness =0.8 mm, 12 averages ,b0 + three seperate directions (FE, PE, SS), b-value = 107 s/mm2.
Diffusion Tensor Imaging (n=6): Images were acquired without diffusion weighting (b0) and then with motion probing gradients applied in 6 different directions.
Analysis Region of interests were drawn around the perivascular tracts surrounding the left and right branch of the MCA, and the subarachnoid space in the mid-section of the Circle of Willis, where previous invasive measures have demonstrated rapid caudal-rostral CSF-tracer movement7,8. The pseudo-diffusion coefficient (D*) was then calculated using the following equation:
S = S0 exp(-bD*)
where S is the measured signal at b=107s/mm2, S0 is the signal taken from the b0 image.
Results
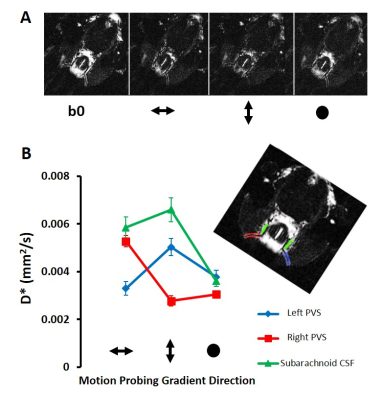
Figure 1D shows a typical b0 image of the axial slice through the ventral aspect of the rat brain. Bright signal is detected from fluid filled compartments, with negligible signal contributions from blood and parenchymal tissue, primarily due to the ultra-long echo time (142 ms) employed. Across the 10 subjects, within the right perivascular space, D*(FE) was significantly greater than D*(PE) [p=0.000007] and D*(SS) [p=0.000001]. In turn, D*(PE) was significantly greater than D*(FE) [p=0.003] and D*(SS) [p=0.005] in the left perivascular compartment (Figure 2).
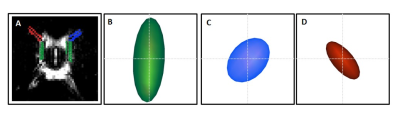
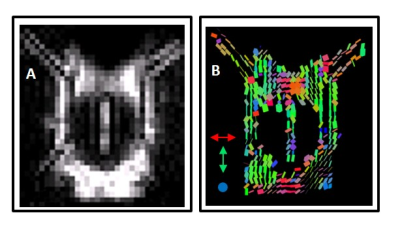
Figure 3 illustrates that the mean D* tensor ellipsoid (n=6) was well aligned with the known principle direction of CSF movement in the subarachnoid space ROI and both the left and right perivascular space respectively7,9,10. Figure 4 shows an example map of pseudo diffusion tensors for a single subject. Together, these data demonstrate that the MRI sequences employed here can detect the directional dependence of fluid movement within the perivascular space (the principal directionality of which is parallel to their orientation), which verifies that they are sensitised to the movement of fluid within this compartment.
Discussion/Conclusion
In this study, we introduce a novel, translational MRI method to measure a distinct feature of brain physiology that, to date, has only be assessed using invasive methods – the movement of fluid in the perivascular space. The perivascular space serves as a preferential pathway for CSF-ISF exchange (e.g. glymphatics), an important mechanism supporting the clearance of potentially harmful molecules, such as Aβ, from the CNS. This technique may expedite greater understanding of how Aβ clearance mechanisms become impaired with ageing11 and in turn reveal a new window in early AD pathogenesis in which to target future diagnostic and treatment strategies.Acknowledgements
This work is supported by the Medical Research Council (MR/K501268/1) and the EPSRC-funded UCL Centre for Doctoral Training in Medical Imaging (EP/L016478/1) together with the Wellcome Trust and Royal Society. DLT is supported by the UCL Leonard Wolfson Experimental Neurology Centre (PR/ylr/18575)References
1. Louveau, A. et al. Nature 523, 337-341 (2015). 2. Iliff, J. J. et al. Science translational medicine 4, 147ra111, (2012). 3. Absinta, M. et al. . eLife 6, e29738, (2017). 4. Ringstad, G., Vatnehol, S. A. S. & Eide, P. K. 140, 2691-2705 (2017) 5. Mawuenyega, K. G. et al. Science 330, 1774, (2010). 6. Xu, Z. et al. . Mol Neurodegener 10, 015-0056 (2015). 7. Iliff, J. J. et al. The Journal of clinical investigation 123, (2013). 8. Lochhead, J. et al.. J Cereb Blood Flow Metab 35, 371-381 (2015) 9. Bedussi, B. et al. . J Cereb Blood Flow Metab 37, 1374-1385,(2017). 10. Lee, H. et al. . Magn Reson Med 19, 26779 (2017). 11. Kress, B. T. et al. Ann Neurol 76, 845-861 (2014)
Figures