0723
Reversal of Behavioral and Metabolic Deficit with Withania Somnifera in Alzheimer's Disease Mice: A 1H-[13C]-NMR Study1NMR Microimaging and Spectroscopy, Centre for Cellular and Molecular Biology, Hyderabad, India
Synopsis
Alzheimer’s disease (AD) is a degenerative disorder and the most common cause of dementia. The hallmark of AD is the accumulation of Aβ-plaque in the subject brain. At present there is no treatment for AD. Extracts of Withania somnifera (WS) roots has been shown to promote neurite outgrowth and improve learning and memory in AD mice. The current study examine the effects of WS on neurometabolism in AβPP-PS1 mouse model of AD by using 1H-[13C]-NMR spectroscopy in conjunction with infusion of [1,6-13C2]glucose. Our findings indicate that WS improved learning and memory, and ameliorate the neurometabolism in AβPPPS1 mice.
Introduction
Alzheimer's disease (AD) is a neurological disorder, which causes memory loss and cognitive decline. The hallmark of the disease is accumulation of Aβ- plaques in the hippocampus and cerebral cortex. At present no effective treatments are available that could reverse the memory and cognitive function in AD. Withania somnifera (WS) is an important herb, contains alkaloids like Withanolide A and withanoside IV, promotes neurite outgrowth in neuronal culture. Moreover, WS promotes longevity, augments defense against disease, improves learning ability and memory capacity1. The objective of the current study was to evaluate the potential of WS on reversal of the behavioral and neurometabolic deficits in AβPP-PS1 mice model of AD by using 1H-[13C]-NMR spectroscopy in conjunction with infusion of [1,6-13C2]glucose.Materials and Methods
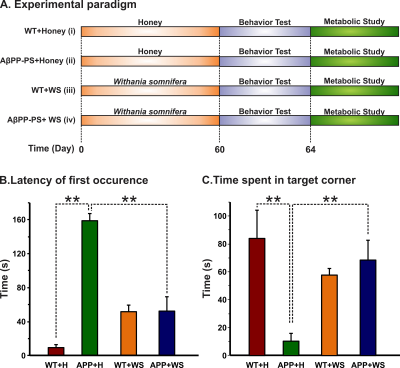
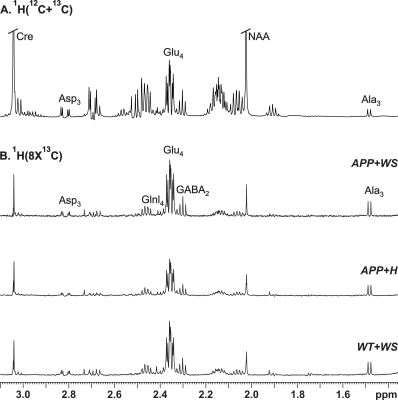
All animal experiments were performed under approved protocols by the Institutional Animal Ethics Committee of CCMB. Eight months old AβPP-PS1 and wild-type (WT) mice were divided into followings: Group (I) WT + Honey (H) (10% in distilled water; n=5); Group (II) AβPP-PS1 + H (n=6); Group (III) WT + WS (20 mg/kg, i.g.; n=6); Group (IV) AβPP-PS1 + WS (n=5). WS roots powder was suspended in honey (10%), and administered orally (20 mg/kg) for 60 days in Group (III) and (IV) mice, while those in Group (I) and (II) received same volume of honey (Figure 1A). Memory of the mice was accessed by Novel Object Recognition Test (NORT)2 (Figure 1B,C). For metabolic analysis, overnight fasted mice were anesthetized using urethane (1.5 g/kg, i.p.), and lateral tail vein was catheterized for infusion of 13C labeled glucose. The body temperature was maintained ~37°C. After 45 min of induction of anesthesia, [1,6-13C2]glucose was administered using a bolus variable infusion rate protocol3. Blood was collected from orbital sinus just before the end of the infusion for analysis of glucose. The head was frozen in situ in liquid nitrogen. The cerebral cortex, hippocampus and cerebellum were dissected, and metabolites were extracted from frozen brain tissues4. The concentration and percentage 13C enrichment of amino acids were measured using 1H-[13C]-NMR spectroscopy in tissue extracts at 600 MHz spectrometer (Figure 2)5. The metabolic rates of glucose oxidation by neurons were determined from the 13C labeling of brain amino acids from [1,6-13C2]glucose as described previously6.Results and Discussion
The learning and memory of mice in the different group were monitored by the Novel Object Recognition Test (NORT). The latency of the first occurrence of AβPP-PS1 (158.5±8.6 s, n=6) mice treated with honey was significantly (p<0.001) high when compared with WT (9.1±3.6 s, n=5), and reduced significantly (p<0.001) following WS treatment (52.4±17.2, n=6, Figure 1B). Moreover, the time spent by AβPP-PS1 mice towards the novel object was increased significantly (p<0.001) after WS treatment (68.6±14.1s, n=6, Figure 1C). These data suggest that WS intervention improved learning and memory in AβPP-PS1 mice.
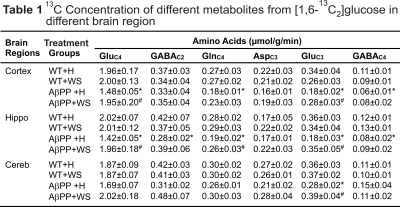
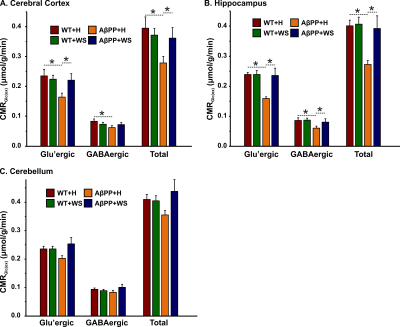
The concentrations of 13C labeled glutamate-C4 (p=0.004), glutamine-C4 (p=0.001) and glutamate-C3 (p=0.001) were decreased significantly in the cerebral cortex in AβPP-PS1 when compared with age-matched controls (Figure 2, Table 1). The rates of glucose oxidation in glutamatergic neurons was found to be reduced significantly (p<0.05) in the cerebral cortex and hippocampus (Figure 3 A,B). Additionally, the reduction in glutamine-C4 labeling suggests decreased synaptic transmission in AβPP-PS1 mice (Figure 2). There was no significant change in the neuronal glucose oxidation in the cerebellum of AβPP-PS1 mice (Figure 3C). Most interestingly, the 13C labeling of glutamate-C4, glutamate-C3 and glutamine-C4 was increased significantly (p<0.05) with WS intervention in AβPP-PS1 mice, which resulted in increased cerebral metabolic rate of glucose oxidation associated with glutamatergic neurons in the cerebral cortex (0.16±0.02, n=6 vs 0.22±0.02 µmol/g/min, n=5, p=0.044), hippocampus (0.16±0.02, n=6 vs 0.24±0.01 µmol/g/min, n=5, p=0.021). These findings together with established stoichiometric coupling between neuronal glucose oxidation and neurotransmitter cycling7 suggest that WS treatment improved synaptic transmission in AβPP-PS1 mice. Hence, WS has potential for improvement of memory and cognitive function in AD.
Acknowledgements
This study was supported by grants from the Department of Science and Technology (AB/013/2013) and CSIR network project BSC0208.References
1. Kaur T, and Kaur G (2017) Withania somnifera as a potential candidate to ameliorate high fat diet-induced anxiety and neuroinflammation, Journal of neuroinflammation 14, 201.
2. Leger M, Quiedeville A, Bouet V, Haelewyn B, Boulouard M, Schumann-Bard P, and Freret T (2013) Object recognition test in mice, Nature protocols 8, 2531-2537.
3. Fitzpatrick SM, Hetherington HP, Behar KL and Shulman RG (1990) The flux from glucose to glutamate in the rat brain in vivo as determined by 1H-observed, 13C-edited NMR spectroscopy, Journal of cerebral blood flow and metabolism 10, 170-179.
4. Patel AB, Rothman DL, Cline GW and Behar KL (2001) Glutamine is the major precursor for GABA synthesis in rat neocortex in vivo following acute GABA-transaminase inhibition. Brain Res 919(2):207-220.
5. de Graaf RA, Brown PB, Mason GF, et al. (2003) Detection of [1,6-13C2]glucose metabolism in rat brain by in vivo 1H-[13C]-NMR spectroscopy. Magn Reson Med 49(1):37-46.
6. Patel AB, de Graaf RA, Mason GF, Rothman DL, Shulman RG, Behar KL (2005) The contribution of GABA to glutamate/glutamine cycling and energy metabolism in the rat cortex in vivo. Proc Natl Acad Sci USA 102:5588-93.
7. Sibson NR, Dhankhar A, Mason GF, Rothman DL, Behar KL, Shulman RG. (1998) Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc Natl Acad Sci USA 95: 316–321.
Figures