0714
Added value of magnetic resonance spectroscopic imaging to multiparametric MRI for detection of prostate cancer using PIRADS version 21Department of NMR and MRI Facility, All India Institute of Medical Sciences, New Delhi, India, 2Department of Radiodiagnosis, All India Institute of Medical Sciences, New Delhi, India, 3Department of Urology, All India Institute of Medical Sciences, New Delhi, India
Synopsis
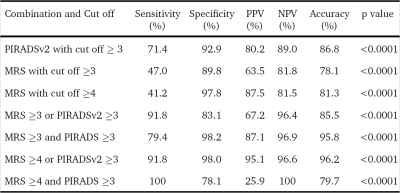
We report increase in sensitivity, specificity and accuracy of PIRADSv2 by addition of MRSI to mpMRI protocol for detection of cancer of prostate (CaP). 26 patients having a biopsy-proven CaP, were investigated at 3.0T using mpMRI including MRSI, followed by radical prostatectomy within 1 month. PIRADSv2 score for mpMRI, PIRADSv1 score for MRSI and Gleason grade group from radical prostatectomy specimens were determined. ROC curve analysis was used to determine the accuracy for cut-offs. A combination of MRSI PIRADSv1 cut-off ≥4 and PIRADSv2 cut-off ≥3 showed the best accuracy (96.2%) for CaP detection. When MRSI was added to PIRADSv2 scoring we found an improvement from 71.4% sensitivity and 91.9% specificity of PIRADSv2 to 91.8% sensitivity and 98% specificity for detecting CaP.
Introduction
Multiparametric MRI (mpMRI) utilizes a combination of T2 weighted (T2W) imaging, diffusion-weighted imaging (DWI) and dynamic contrast-enhanced (DCE) MRI for evaluation of cancer of prostate (CaP). To standardize the reporting of lesion appearing on mpMRI, a structured mpMRI reporting system, prostate imaging reporting, archiving and data system version 2 (PIRADSv2) was introduced (1). Most of the studies evaluating PIRADSv2 used systematic or targeted biopsies as reference standards and all of them are retrospective studies making use of data before the publication of PIRADSv2. Therefore, the present study was carried out prospectively with radical prostatectomy specimens as the reference standard to evaluate the addition of magnetic resonance spectroscopic imaging (MRSI) to mpMRI using PIRADSv2 for detection of CaP.Methods
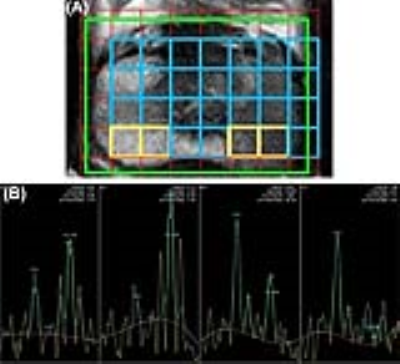
A total of 26 patients (range, 48-75 years, mean±SD, 63.3±7 years), having a biopsy-proven CaP, were investigated using mpMRI including MRSI, followed by radical prostatectomy within 1 month. MR investigations were carried out at 3.0T MRI scanner (Ingenia, Philips, Netherlands) using a phased array body coil. MRI protocol consisted of T2W (axial, coronal and sagittal planes) and T1W (axial) imaging, DWI (single-shot echo-planar imaging sequences; b-values 0, 500, 1000 and 1500 s/mm2, DCE-MRI and MRSI. MRSI was performed using a 3D multi-voxel PRESS sequence with water and fat suppression. For DCE-MRI, T1 maps were acquired using a multiphase rapid T1-weighted spoiled gradient-echo sequence. Gadodiamide, 0.1 mmol/kg, was administered intravenously at a rate of 3ml/sec. Prostate images were divided into 16 sectors (12, peripheral zone (PZ) and 4, transitional zone (TZ) sectors) per patient. Each of these sectors was assigned a PIRADSv2 score for T2WI, DWI and DCE-MRI images individually, followed by an overall score for the sector. A single best voxel with the highest choline peak was chosen for each of the sectors and the (choline+creatine)/citrate ratio (CCC ratio) was determined (Fig.1). MRSI PIRADSv1 score was separately recorded for each of the included sectors because PIRADSv2 does not provide any recommendations for interpreting MRSI. The radical prostatectomy specimen was sectioned into 3mm cuts, in the axial plane to determine the Gleason grade group.Results
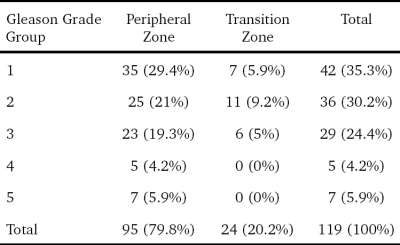
The serum prostate specific antigen (PSA) values ranged from 5.9 to 23.2 ng/ml (mean, 12 ± 5 ng/ml). Out of the 416 sectors which were assessed [312 PZ + 104 TZ], 119 (28.6%) were positive for cancer on histopathology. 95 of these 119 positive sectors (79.8%) were from the PZ while 24 (20.2%) of them were from the TZ. The median number of cancer-positive sectors per prostate was 2.5 (range 1-16). The proportions of various grades among the cancer positive sectors are summarized in Table 1. ROC curve analysis of the MRSI PIRADSv1 score of the 310 sectors showed an AUC of 0.74 (CI 0.67-0.81). MRSI data from 106 sectors were excluded due to low quality. The accuracy for various cut-offs and combinations with PIRADSv2 is summarized in Table 2. Among the various permutations used, a combination of MRSI PIRADSv1 cut-off ≥4 and PIRADSv2 cut-off ≥3 in a parallel configuration (i.e., a test is taken as positive if either test is positive) showed the best accuracy (96.2%) for CaP detection. This included 28.6% (n = 34/119) of the studied cancer positive sectors.Discussion
In this study, we assessed the added role of MRSI to the PIRADSv2 recommended mpMRI protocol. MRSI alone (using PIRADSv1 assessment criteria) showed sensitivity, 47%, and specificity, 89.8% for ≥3 score cut-off (sensitivity, 41.2%, and specificity, 97.8%, for ≥4 score cut-off). However, when MRSI was added to PIRADSv2 scoring and used both tests in parallel we found an improvement from 71.4% sensitivity and 91.9% specificity of PIRADSv2 to 91.8% sensitivity and 98% specificity for detecting CaP. There are only a few studies that analyzed the MRSI component of PIRADSv1. Kuru et al., using systematic biopsy as reference standard showed a sensitivity and specificity of 76% and 83%, respectively, for MRSI PIRADSv1 scoring as a stand-alone (2). Meier-Schroers et al. using MR guided biopsy, showed a sensitivity and specificity of 43% and 84%, respectively, for PIRADSv1 sun score including MRSI (3).Conclusion
Our study revealed that addition of MRSI to mpMRI protocol might increase the ability of PIRADSv2 scoring system for detection of CaP.Acknowledgements
No acknowledgement found.References
1. Weinreb JC et al. PI-RADS Prostate Imaging-Reporting and Data System: 2015, Version 2. Eur Urol. 2016;69:16–40
2. Kuru TH et al. Histology core-specific evaluation of the European Society of Urogenital Radiology (ESUR) standardized scoring system of multiparametric magnetic resonance imaging (mpMRI) of the prostate. BJU Int. 2013;112:1080–7
3. Meier-Schroers et al. Differentiation of prostatitis and prostate cancer using the Prostate Imaging—Reporting and Data System (PI-RADS). Eur J Radiol. 2016;85:1304–11
Figures