0712
Simultaneous Acquisition reveals Functional Connectivity in Brain and Spinal Cord1Stanford University, Palo Alto, CA, United States
Synopsis
We investigate resting-state brain and spinal cord functional connectivity of four healthy volunteers by means of a novel dynamic per slice shimming approach. Functional connectivity, between brain and spinal cord, reveals localization to corresponding sensory and motor areas. Our results are consistent with task-based brain-spinal cord activation results using a motor task , suggesting a strong connection of descending and ascending signals with the brain.
Introduction
Functional connectivity within the brain has been studied extensively using fMRI. But limiting investigation to only the brain provides a truncated view of the central nervous system (CNS) as it does not capture information exchange between brain and spinal cord. Simultaneous brain-spinal cord fMRI provides a means to measure connectivity across CNS but is challenging for many reasons; foremost: 1) field inhomogeneity in spinal cord induces image distortion and signal loss, 2) spinal cord motion from cardiac and respiratory activity produces unwanted signal fluctuation while inhibiting registration, 3) small size of spinal cord (~1cm) requires long readout for adequate spatial resolution. [1] Due to these technical challenges, functional connectivity between brain and spinal cord remains largely unexplored. In this study, we investigate resting-state brain and spinal cord functional connectivity of four healthy volunteers by means of a novel dynamic per slice shimming approach to minimize field inhomogeneity [2-3], and we apply an echo-planar RF pulse [4] to selectively excite spinal cord while avoiding aliasing under reduced FOV. We use parallel imaging for shorter readouts, but incorporate predetermined and different readout gradients to obtain brain resolution different from that for spinal cord.Methods
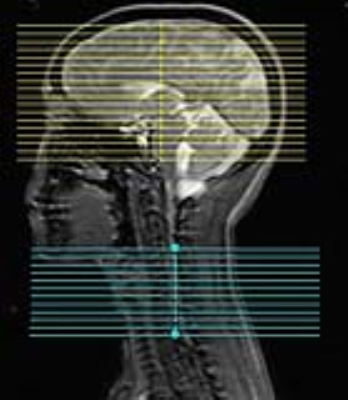
Methods: Using a dynamic shimming procedure previously reported [2-3], resting-state scans were collected at a GE 3T Discovery 750 scanner. The fMRI sequence parameters were: EPI GRAPPA (R=2) [5], flip angle=80°, FOV for brain/spinal cord = 22cm/8cm, matrix size=64x64, readout BW=±125 kHz, TE/TR=30ms/2.4s, #slices=30 (18 brain, 12 spinal in C5-C8 region), slice thickness/spacing=4mm/2mm (see Figure 1). We use slice-select excitation pulse in brain slices and an echo-planar pulse in spinal cord slices. Volunteers are instructed to lie still in the scanner with eyes closed, to not fall asleep, and to not think of anything in particular. Two 10-minute resting-state scans (together with cardiac and respiratory data) are collected from each volunteer. Physiological noise is removed from brain and spinal cord images using RETROICOR [6]. Images are corrected for slice timing and motion, after which, mean CSF and white matter signals are removed using custom software. Images are further bandpass filtered (0.01-0.0.198 Hz), spatially normalized to standard space (MNI152 template for brain, PAM50 for spine), then spatially smoothed (5×5×5 mm3 FWHM Gaussian kernel for brain, 2×2×6mm3 kernel for spine). The mean time series, from left-ventral, left-dorsal, right-dorsal, and right-ventral spinal cord horn at the C5, C6, and C7 spinal cord segments, are extracted from processed spinal cord images, and then used to generate subject-level connectivity maps for brain and spinal cord with FSL. Connectivity maps, from the two runs, are averaged (fixed-effects) then average group-level connectivity maps are generated (mixed effects FLAME1+2, cluster forming threshold Z>1.64, and FWE corrected cluster size threshold p<0.05).Results
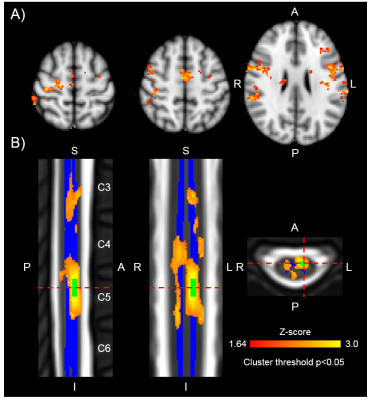
Functional connectivity, between brain and spinal cord, reveals localization to corresponding sensory and motor areas. Brain connectivity to C6 left-ventral horn, for example, encompasses the right motor cortex, right somatosensory cortex, bilateral supplementary motor cortex, and bilateral insular cortices, whereas spinal cord connectivity is localized, primarily, to the C6 left-ventral horn (Figure 2).Discussion
Resting-state functional connectivity of the brain has already provided valuable insight into psychiatric disorders (bipolar, depressive, dementia), disease (Parkinson, stroke), and pain. [7] Our contribution augments the broader study of connectivity by providing a new tool for observation of simultaneous brain-spinal cord connectivity; namely, a brain-spinal cord pulse sequence. Connectivity observed in this study is consistent with neuroanatomical connections of corticospinal sensorimotor pathways. Furthermore, these results are consistent with task-based brain-spinal cord activation results using a motor task [2-3], suggesting a strong connection of descending and ascending signals with the brain. Future studies will explore dynamics of functional connectivity between brain and spine in health and disorder.Acknowledgements
General Electric Healthcare. NIH Grant: P41 EB0015891, R01 NS053961, K24 DA029262, NIDA T32 DA035165. Ambhir-RSL Innovation Challenge GrantReferences
1. Stroman PW, et al. The current state-of-the-art of spinal cord imaging: Methods. Neuroimage 2014;84:1070–1081.
2. Haisam I, et al. Simultaneous brain and spinal cord fMRI using slice-based dynamic shimming. ISMRM 2017 Honolulu: 1634.
3. Haisam I, et al. Simultaneous brain and spinal cord fMRI using slice-based dynamic shimming. Submitted to MRM. Under revision.
4. Pauly J, et al. A k-space analysis of small-tip-angle excitation. J Magn Reson 1989;81:43–56.
5. Griswold MA, et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med 2002; 47:1202–1210.
6. Glover GH, et al. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2000;44:162-7.
7. Takamura T, et al. Clinical utility of resting-state functional connectivity magnetic resonance imaging for mood and cognitive disorders. Journal of Neural Transmission. 2017;124(7):821-839.
Figures