0711
Band-free whole-brain alternating SSFP fMRI provides distortion-free activation maps in the visual cortex at 7T1Center for Biomedical Imaging (CIBM), EPFL, Lausanne, Switzerland
Synopsis
Although the potential of bSSFP for fMRI has been previously demonstrated, the use of bSSFP remains limited at high field due to the presence of banding artefacts. We demonstrate here that a modified bSSFP sequence with alternating phase cycles (0/180°) can provide distortion-free and band-free fMRI images at 7 Tesla with a wide coverage (72 mm), and temporal / spatial resolution that match that of conventional EPI (2 mm isotropic, TRvolume=1.7 s), by combining water-selective spectro-spatial pulses and very high undersampling (12-fold) coupled with controlled aliasing (CAIPIRINHA).
Introduction
Balanced steady-state free precession (bSSFP) techniques1 provide high signal-to-noise ratio and can constitute an excellent alternative to GE-EPI for functional studies2-5, especially in highly inhomogeneous regions and/or at high field6. However, the use of SSFP fMRI remains limited due to the presence of transition bands in functional images. By combining bSSFP images acquired with various phase cycling (PC), alternating SSFP (alt-SSFP) can in principle provide band-free images for fMRI with near whole-brain coverage, as previously demonstrated for structural imaging7 and fMRI8 at 3T. Because alt-SSFP fMRI requires the settling of a pseudo steady-state within a timescale small enough to probe hemodynamic changes, problems related to energy deposition and spin dynamics arise for higher field strengths. In this study, we demonstrate that alt-SSFP is feasible at 7T with near-whole-brain coverage using a combination of parallel imaging9-11, water-selective spectro-spatial pulses12-14 and a judicious choice of acquisition parameters.Methods
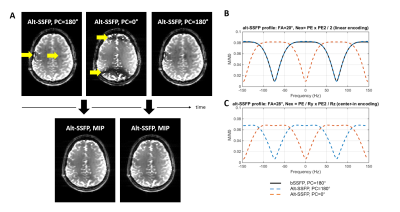
Based on Bloch equations, the spectral profile of the alt-SSFP signal (two phase cycles, PC=0° and 180°) was simulated, compared to the conventional bSSFP profile, and optimized to obtain a flat combined signal profile (Figure 1) when using acceleration factors compatible with fMRI repetition times (TRvolume=1.7s).
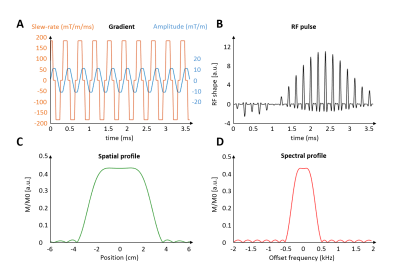
Spectro-spatial (SPSP) pulses12-14 were designed using the Matlab RF_tools toolbox15 to selectively excite water (and not fat) at 7T, while keeping energy deposition low enough to allow the use of moderate flip angles (FA=28°).
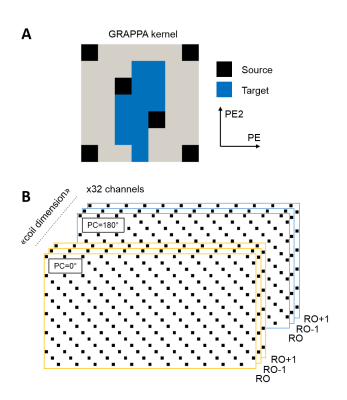
Extended coverage (slab thickness = 72mm) was achieved by implementing GRAPPA9 with controlled aliasing10 (CAIPIRINHA) for 3D data acquisition as in [11] (acceleration 2x6 along PExPE2, with a field-of-view shift of 2, see Figure 2A-B). Reconstruction was performed offline using Matlab, and further enhanced by treating both phase-cycling and neighboring read-outs k-spaces as additional coil sensitivities (Figure 2B). Fully sampled data with similar phase cycling were used for robust GRAPPA calibration (acquisition time TA=34s).
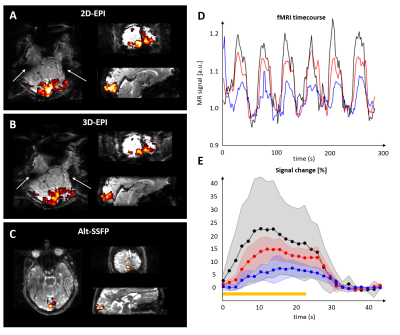
fMRI experiments were performed at 7T using a 1*Tx/32*Rx array coil (Nova). For validation, n=3 subjects underwent visual stimulation for 5 min (17.2s + 6x22.4s [ON/OFF] 2Hz flashing checkerboard), and scanned using 2D-EPI, 3D-EPI (with similar TE=22ms) and 12-fold accelerated alt-SSFP (TE=3.4ms) with similar resolution (2mm isotropic), brain coverage (72 mm slab incorporating the eyes and the visual cortex) and temporal resolution (TRvolume=1.72s). Statistical maps were derived using SPM12, and the average time-series in the 10 voxels with most activation computed after high-pass filtering.
Results
A minimal TRSSFP of 6.8 ms was necessary for specific absorption rate (SAR) limits at 7T. By switching the accelerated alt-SSFP read-out to center-in encoding, a pseudo steady-state was rapidly re-established every TRvolume, with moderately higher flip angle compared to bSSFP (Figure 1B-C, optimal alt-SSFP FA=28° for TRSSFP/TRvolume=6.8/1720 ms, and T1/T2=2000/55 ms). Using parallel imaging, 12-fold accelerated alt-SSFP resulted in a 72 mm slab coverage every 1.7s (Figure 2B, PE2=36).
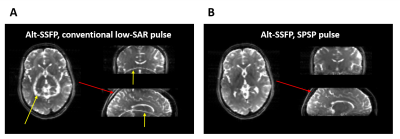
To minimize energy deposition, 4ms SPSP RF pulses were distributed along 19 gradient cycles (Figure 3). Experimentally, SPSP pulses resulted in strong fat suppression (Figure 4A-B) and good spatial localization.
Robust activation was detected in the visual cortex with 2D-, 3D-EPI and alt-SSFP (Figure 5A-C). The average BOLD amplitude was 12, 24 and 7.5% for 2D, 3D-EPI and alt-SSFP, respectively (Figure 5D-E).
Discussion and conclusion
We successfully implemented alt-SSFP with extended brain coverage at 7T, which is highly advantageous in regions of high susceptibility gradients at high field.
Energy deposition was kept to an acceptable
level (SAR < 50% for all subjects) so that this work could be translated to areas suffering from lower transmit field. Alternatively, parallel transmission could be used to further reduce the SAR when needed.
Tailored RF pulses and gradients were necessary to acquire uncontaminated water signal in the brain. The sparsity of the bSSFP signal7 combined with the implementation of controlled aliasing10 permitted high undersampling (GRAPPA=2x6), another requirement to adequately probe temporal dynamics.
Contrary to EPI, a reference data set with similar contrast (ie. phase-cycling) was deemed essential in reconstructing highly undersampled bSSFP data. On the other hand, “dummy”/catalyzation RF pulses could be discarded after switching to center-in encoding along PE with minimal impact on data quality.
Since establishing a pseudo-steady state was mandatory for optimal combination of phase-cycling, this prevented further reducing the repetition time below TRvolume=1.72s for alt-SSFP. However, multiple read-outs8 and/or thinner slabs could be used to perform high spatial resolution alt-SSFP fMRI at high field with similar temporal resolution.
This study opens new and exciting perspectives for laminar fMRI and/or investigating hemodynamics with ultra-high-field fMRI in areas most difficult to probe with GE-EPI, such as the retina, orbitofrontal cortex, olfactory bulb, auditory pathway, optical nerve, lower cerebellum or lower brain regions.
Acknowledgements
This work was supported by the Centre d'Imagerie Bio-Médicale (CIBM) of the University of Lausanne (UNIL), the Swiss Federal Institute of Technology Lausanne (EPFL), the University of Geneva (UniGe), the Centre Hospitalier Universitaire Vaudois (CHUV), the Hôpitaux Universitaires de Genève (HUG) and the Leenaards and the Jeantet Foundations.References
1. Bieri O, Scheffler K. Fundamentals of balanced steady state free precession MRI. J Magn Reson Imaging. 2013 Jul;38(1):2-11. doi: 10.1002/jmri.24163.
2. Zhong, K, Leupold, J, Hennig, J, Speck, O, 2007. Systematic investigation of balanced steady-state free precession for functional MRI in the human visual cortex at 3 Tesla. Magn Reson Med 57, 67-73.
3. Lee JH, Dumoulin SO, Saritas EU et al., 2008. Full-brain coverage and high-resolution imaging capabilities of passband b-SSFP fMRI at 3T. Magn Reson Med 49, 1099-110.
4. Muir, E.R., Duong, T.Q., 2011. Layer-specific functional and anatomical MRI of the retina with passband balanced SSFP. Magn Reson Med 66, 1416-1421.
5. Miller, K.L., 2012. FMRI using balanced steady-state free precession (SSFP). Neuroimage 62, 713-719.
6. Park, S.H., Kim, T., Wang, P., Kim, S.G., 2011. Sensitivity and specificity of high-resolution balanced steady-state free precession fMRI at high field of 9.4T. Neuroimage 58, 168-176.
7. Hilbert T, Nguyen D, Thiran JP, Krueger G, Kober T, Bieri O. True constructive interference in the steady state (trueCISS). Magn Reson Med. 2017 Jul 24. doi: 10.1002/mrm.26836
8. Jou T, Patterson S, Pauly JM, Bowen CV. Fat-suppressed alternating-SSFP for whole-brain fMRI using breath-hold and visual stimulus paradigms. Magn Reson Med. 2016 May;75(5):1978-88. doi: 10.1002/mrm.25797.
9. Griswold M, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, and Haase A. Generalized Autocalibrating Partially Parallel Acquisitions (GRAPPA). Magnetic Resonance in Medicine 47:1202–1210 (2002).
10. Breuer F, Blaimer M, Griswold M, Jakob PM. Controlled Aliasing in Parallel Imaging Results in Higher Acceleration (CAIPIRINHA) Magn Reson Med. 2005 Mar;53(3):684-91.
11. Narsude M, Gallichan D, van der Zwaag W, Gruetter R, Marques JP. Three-dimensional echo planar imaging with controlled aliasing: A sequence for high temporal resolution functional MRI. Magn Reson Med. 2016 Jun;75(6):2350-61. doi: 10.1002/mrm.25835.
12. Meyer CH, Pauly JM, Macovski A, Nishimura DG. Simultaneous spatial and spectral selective excitation. Magn Reson Med 1990; 15:287–304.
13. Block W, Pauly JM, Kerr A, Nishimura DG. Consistent fat suppression with compensated spectral-spatial pulses. Magn Reson Med 1997; 38:198–206.
14. Yuan J, Madore B, and Panych LP. Fat-Water Selective Excitation in Balanced Steady-State Free Precession using Short Spatial-Spectral RF Pulses. J Magn Reson. 2011; 208(2): 219–224. doi:10.1016/j.jmr.2010.11.005.
15. Toolbox available at https://github.com/agentmess/Spectral-Spatial-RF-Pulse-Design
Figures