0709
Blood-volume imaging using GRASE-VASO at ultra-high field for layer specific fMRI in human brain1Helen Wills Neuroscience Institute, University of California, Berkeley, Berkeley, CA, United States, 2Center for Imaging of Neurodegenerative Diseases, Veteran Affairs Health Care System, San Francisco, CA, United States, 3Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 4Department of Radiology, Harvard Medical School, Boston, MA, United States, 5Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, United States, 6Advanced MRI Technologies, LLC, Sebastopol, CA, United States
Synopsis
Cortical layer-dependent fMRI opens new possibilities for studying neuronal circuitry. Gradient-echo BOLD contrast, which is commonly used for fMRI, suffers from decreased spatial specificity due to BOLD contrast arising in large draining veins. Vascular space occupancy (VASO) and gradient and spin echo (GRASE) acquisition techniques were shown to improve spatial specificity. Here, the technique to acquire BOLD-corrected GRASE-VASO images and its application to fMRI in human motor cortex at 7T are presented. The results suggest increased spatial specificity as compared to EPI-VASO, which could be beneficial for layer-dependent fMRI applications.
Introduction
Cortical layer-dependent functional magnetic resonance imaging (fMRI) opens new possibilities for studying neuronal circuitry. Gradient-echo (GE) BOLD contrast, commonly used for fMRI, arises from capillaries as well as from larger draining veins [1], decreasing the spatial specificity of measured neuronal activity. Vascular space occupancy (VASO) images cerebral blood volume (CBV) by nulling blood signal to indirectly measure the volume of vascular compartment. VASO has been shown to be less sensitive to the signal in draining veins, improving specificity.
The residual T2* contrast in VASO counteracts the CBV signal changes in fMRI, so an image with BOLD contrast, interleaved with the VASO image, can correct for residual T2* influence [2]. Gradient and spin echo (GRASE) fMRI at ultra-high magnetic fields has been shown to have higher specificity than GE to the microvasculature and higher sensitivity than spin echo (SE) [3]. Combining BOLD-corrected VASO labeling with GRASE readout may improve cortical depth-dependent fMRI even further.
Here we present simultaneous acquisition of GRASE-VASO and GRASE-BOLD contrasts at 7T in human motor cortex. Cortical depth dependent functional response is investigated and compared to GE echo planar imaging (EPI) acquisition.
Methods
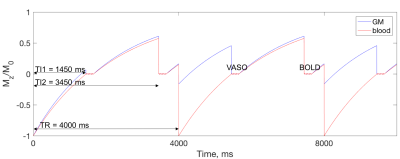
Functional MRI data was acquired in three volunteers at Siemens 7T scanner using a 32-channel Nova head coil. 3D GRASE and 2D single-shot EPI pulse sequences were modified to include a non-selective IR pulse for the VASO contrast. In addition, a second acquisitions with BOLD contrast was interleaved with VASO acquisition (Fig. 1). BOLD image in both GRASE and EPI was used to correct for residual T2* effect [2]. The following parameters were used for zoomed-GRASE acquisition [4]: TI1/TI2/TR/TE = 1450/3450/4000/41 ms, FOV = 128×32 mm and matrix = 128×32, yielding 1 mm isotropic resolution. GRASE factors = SE/5 & GE/32, slice-axis partial Fourier of 5/8, and centric order on slice axis were used. For EPI acquisition: TI1/TI2/TR/TE = 1450/3450/4000/21 ms, FOV = 192×192 mm, matrix = 192×192, yielding 1 mm isotropic resolution. Phase-axis partial Fourier of 5/8 and iPAT = 2 were used.
Functional activation was identified using a finger tapping task, with left and right finger tapping alternating every 16 seconds leading to robust activation in left and right M1. Correlation analysis implemented in Matlab was used to identify the activated voxels. Functional images were co-registered with whole-head MPRAGE anatomical image, which was used to generate white-matter and pial surfaces using Freesurfer [5]. These surfaces were used to generate ROIs in deep, middle and superficial gray-matter within left and right M1, and the mean signal change in response to alternating left and right finger tapping was calculated for each scan type.
Results
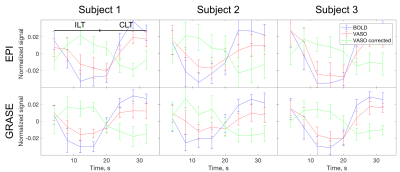
Signal time course during activations in M1 for BOLD, VASO, and corrected VASO is shown at Fig. 2. Before the correction, VASO signal changes in the same direction as BOLD. The signal in corrected VASO images, however, changes is opposite to BOLD direction.
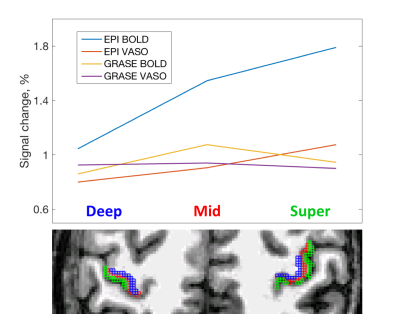
The cortical depth dependent signal change is presented in Fig. 3. EPI-BOLD has the highest amplitude that peaks in superficial GM. EPI-VASO has a similar behavior, although signal change is more evenly distributed across the cortex. For the GRASE acquisition, both BOLD and VASO signal change have slightly lower amplitude in superficial GM.
Discussion
The signal change for BOLD, VASO, and corrected VASO is consistent between three subjects for both EPI and GRASE readouts (Fig. 2). Signal in uncorrected VASO is underestimated due to the residual BOLD signal. In corrected VASO the signal change is in an opposite direction to BOLD, which is consistent with increase in blood volume during activations.
The results of depth-dependent signal change for EPI-VASO are in agreements with previously published [6]. In GRASE the effect of superficial large draining veins is reduced for both BOLD and VASO contrast, as compared to EPI, yielding higher spatial specificity. This allows identifying slightly higher signal change in the middle of the cortex, which is consistent with arising from the micro- rather than macro-vasculature, and hence being more layer specific. Further improvement should be achieved by optimizing the inversion timing for higher SNR in VASO images and developing sequence and hardware improvement to push for higher resolution.
Conclusion
BOLD-corrected VASO acquired using GRASE readout can be successfully used at 7T for fMRI. The signal change observed in the activation regions is comparable to BOLD-corrected EPI-VASO. The cortical depth-dependent signal profile of GRASE-VASO suggests that the signal is less sensitive to the contribution of large veins in superficial GM layers than in EPI-VASO. The improved spatial specificity of GRASE-VASO is potentially beneficial for layer-dependent fMRI studies of neuronal circuitry and columns.Acknowledgements
Supported by NIH 1U01EB025162-01, 1R24MH106096, R01MH111419, 5R01MH111444. Authors would like to thank Laurentius Huber for the useful discussions and Matthias Guenther for the help with the pulse sequence.References
[1] Turner R, 2002. How much cortex can a vein drain? Downstream dilution of activation-related cerebral blood oxygenation changes. Neuroimage 16(4), 1062-1067
[2] Huber L, Ivanov D, Krieger SN, Streicher MN, Mildner T, Poser BA, Möller HE, Turner R, 2013. Slab-selective, BOLD-corrected VASO at 7 Tesla provides measures of cerebral blood volume reactivity with high signal-to-noise ratio. Magn Reson Med 72(1), 137-148
[3] De Martino F, Zimmermann J, Muckli L, Ugurbil K, Yacoub E, Goebel R, 2013. Cortical depth dependent functional responses in humans at 7T: improved specificity with 3D GRASE. PLoS One 8(3), e60514
[4] Feinberg DA, Harel N, Ramanna S, Ugurbil K, Yacoub E, 2008. Sub-millimeter Single-shot 3D GRASE with Inner Volume Selection for T2 weighted fMRI applications at 7 Tesla. Proc Intl Soc Mag Reson Med 16, 2373
[5] Dale AM, Fischl B, Sereno MI, 1999. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 9, 179-194
[6] Huber L, Goense J, Kennerley AJ, Trampel R, Guidi M, Reimer E, Ivanov D, Neef N, Gauthier CJ, Turner R, Möller HE, 2015. Cortical lamina-dependent blood volume changes in human brain at 7T. Neuroimage 107, 23-33
Figures