0707
Functional sodium (23Na) MRI at 7T - Extracellular sodium decreases during cortical activation in the Human brain1Aix-Marseille Univ, CNRS, CRMBM, Marseille, France, 2AP-HM, Timone Uinv Hospital, CEMEREM, Marseille, France, 3Computer Assisted Clinical Medicine, Centre for Biomedicine and Medical Technology Mannheim, Heidelberg University, Mannheim, Germany
Synopsis
Using dynamic multiecho 3D 23Na MRI with a temporal resolution of 25s, we demonstrated that functional 23Na MRI at 7T was sensitive enough to observe non-invasively in the Human brain, sodium signal variations during a conventional hand motor task. This acquisition performed at 3 different TEs showed that the closest spatial pattern of sodium signal changes relative to BOLD activation was the decrease of 23Na signal at long TE (19ms) assuming to mostly reflect the extracellular sodium changes. This work opens a new ear to better understand normal and abnormal activity-dependent metabolic coupling in the neuro-glia-vasculature ensemble.
Introduction
During the last decade, paralleled with hardware/sequence improvement and clinical availability of high field and ultra high field MR scanners1, brain sodium (23Na) MRI has regained interest for the neurological community to characterize dysfunction of ionic homeostasis in various diseases2. However, the poor sensitivity of the technique has not allowed yet to access one major phenomenon of ionic homeostasis involved in brain function, i.e. the ability to monitor sodium dynamics following cortical activation. Here we propose a dynamic 23Na MRI approach enabling to non invasively observe the relative sodium signal changes following a simple right hand movement within the different brain compartments.Methods
Multi-echoes 3D- radial density adapted 23Na-MRI 4 was acquired in 12 right handed healthy controls on a Magnetom 7T MR scanner (Siemens, Erlangen, Germany) using a dual tuned QED 1H/23Na birdcage coil with the following acquisition parameters (TE1/2/3=0.2ms/10ms/19ms; TR=120ms, 10080 spokes, isotropic voxel = (3mm)3, BW 280 Hz/pixel, density adapted (t0 = 500 us), TA=20min) (Figure 1). Successive spoke angles were incremented by the golden angle and interleaved by a factor of 42 to give a smooth azimuthal variation through k-space and full polar coverage every 25s 5 (Figure 2). This allowed to reconstruct temporal series of 42 23Na MR volumes. An opposition finger task of the right was performed with 21 alternations of rest/active periods during this dynamic acquisition (one volume acquired per condition every 25s). After denoising (rician filter), spatial realignment and normalization, smoothing (FWHM=9mm), 3D volumes were entered into a GLM to detect signal changes during right hand movement for each TE. Second level group analyses were done for each individual TE, and also a within subject ANOVA was performed to account for all TEs of individual subjects (SPM12, p<0.02, k=5, FDR corrected). BOLD 1H-fMRI (5 alternations of rest/active periods of 20 measurements per condition) using multiband EPI sequence (TE=20ms, TR=1s, 85 slices, MB=3, grappa 2, isotropic voxel = (1.6mm)3) was used as reference for activation patterns and conventional GLM post-processing was applied onto the 200 measurements (SPM12, p<0.02, k=5, FDR corrected).Results
BOLD activation patterns and variations of 23Na signals for each TE are displayed on Fig 3 (increases) and Fig 4 (decreases). Significant sodium signal variations were observed within the motor network. The best correspondence of 23Na signal variations with the BOLD activation pattern during right hand movement was observed for the decrease of 23Na signal at TE=19ms.Discussion
Imaging Na+ changes dynamically is a breakthrough in brain functional imaging. So far, such information has not been addressed in vivo and non-invasively. We demonstrate here that non-invasive observation of sodium dynamics following cortical activation is feasible in the human brain using 23Na fMRI. The best patterns of 23Na signal changes corresponded to a decrease of the long T2 component sodium, mostly reflecting a decrease in extracellular concentrations in accordance with invasive studies in animal models6,7.Conclusion
This study may open a new era in the better understanding of the normal activity-dependent metabolic coupling in the neuro-glia-vasculature ensemble3, and decoupling in neurological diseases.Acknowledgements
This work was supported by the following funding sources: Investissements d’Avenir 7T-AMI-ANR-11-EQPX-0001, A*MIDEX-EI-13-07-130115-08.38-7T-AMISTART. Aix-Marseille Université, AP-HM and CNRS (Centre National de la Recherche Scientifique).References
1. Shah NJ et al. NMR Biomed. 29(2):162-74, 2016. 2. Thulborn KR Neuroimage S1053-8119(16)30674-7, 2016. 3. Jolivet R et al. PLoS Comput Biol. 26;11(2):e1004036, 2015 4. Nagel A et al. Magn Reson Med. 62(6):1565-73, 2009 5. Bydder M et al. ISMRM #4312, 2017 6. Dietzel et al Exp Brain Res 46(1):73-84. 1982. 7. Haack et al. J Vis Exp 5; (103). 2015.Figures
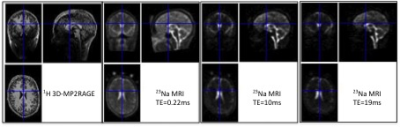
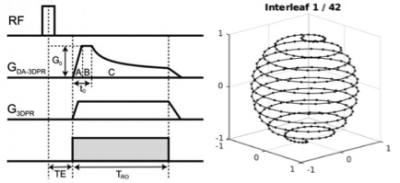
Figure 2: Density adapted 3D projection reconstruction sequence (Nagel 2009) with 10080 spokes
Radial spokes are incremented by the golden angle and re-ordered into 42 interleaves to give full azimuthal and polar coverage every 240 spokes (25s).
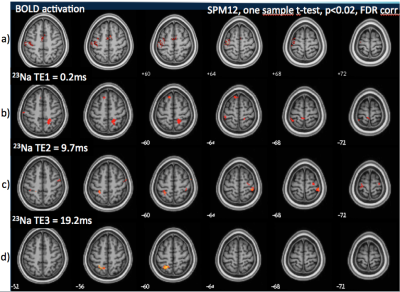
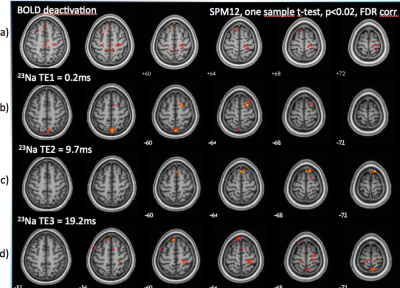
Figure 4: BOLD deactivation patterns and decreases in sodium signal during right hand movement (One sample t-test, p<0.02, k=5, FDR corrected).
a) BOLD deactivation was observed in the right PMA and bilateral precuneus. b) At short TE of 0.2ms, 23Na signal decreases in the right SFG, precuneus and (not shown) the right insula and the left cerebellum. c) At TE=10ms, 23Na signal decreases in the supplementary motor cortex. d) at TE of 19ms, 23Na signal decreases in the bilateral precentral gyri, the SMA, the right superior parietal lobule superior parietal lobule, the left precuneus, and (not shown) the right cerebellum.