0687
Highly accelerated 4D flow in the aorta with compressed sensing, respiratory controlled adaptive k-space reordering and inline reconstructionNing Jin1, Liliana Ma2,3, Kelvin Chow2,4, Christoph Forman5, Andreas Greiser5, Susanne Schnell2, Alex Barker2, and Michael Markl2,3
1Cardiovascular MR R&D, Siemens Medical Solutions USA, Inc., Columbus, OH, United States, 2Department of Radiology, Feinberg School of Medicine, Northwestern University, Chicago, IL, United States, 3Department of Biomedical Engineering, Northwestern University, Chicago, IL, United States, 4Cardiovascular MR R&D, Siemens Medical Solutions USA, Inc., Chicago, IL, United States, 5Siemens Healthcare, Erlangen, Germany
Synopsis
The clinical application of 4D flow is limited by its long acquisition time. Recently, sparse sampling and compressed sensing (CS) reconstruction have been combined with 4D flow to speed up the image acquisition. However, most of CS reconstructions are implemented offline with long reconstruction times, making them infeasible for use in clinical settings. We developed a highly accelerated 4D flow with CS and Respiratory Controlled Adaptive k-space Reordering (ReCAR) acquisition to enable a 3-minute aortic protocol with isotropic 2.5mm voxels and fast 5-minute inline reconstruction. Initial volunteer tests show a good match of flow quantification results with the conventional technique.
Introduction
4D flow MRI enables the visualization of complex 3D flow patterns in three-dimensional vasculature through the entire cardiac cycle and provides feasibility of retrospective flow analysis at any location in the imaging volume1. Despite the advantages provided by 4D flow imaging, widespread clinical application is still limited due to the long acquisition time intrinsic to multi-dimensional imaging (3D spatial encoding + time + 3-directional velocity encoding). Parallel acceleration techniques, like GRAPPA or SENSE, have been commonly applied to 4D flow MRI to provide 2 to 3-fold acceleration. K-t acceleration techniques, such as PEAK-GRAPPA2 further exploit the spatiotemporal correlation to achieve a rate 5 acceleration. Recently, sparse sampling and compressed sensing (CS) reconstruction have been combined with 4D flow to achieve even higher acceleration factors3-6. However, most CS reconstructions are implemented offline with long reconstruction times, making them infeasible for use in clinical settings. The purpose of this study was to develop a free-breathing highly accelerated 4D flow imaging suitable for clinical workflow using L1-regularized wavelet-based CS and Respiratory Controlled Adaptive k-space Reordering (ReCAR)7-8. Aortic 4D flow images could be acquired within three minutes with inline image reconstruction taking less than five minutes.Methods
CS 4D flow data was acquired during free-breathing using a prototype navigator (NAV) gated 3D cine phase contrast sequence with interleaved three-directional velocity encoding. ReCAR was based on the respiratory position as detected by the navigator to acquire the central k-space at end-expiration with minimal respiratory displacement and the outer k-space during inspiration. A variable density spiral phyllotaxis pattern was used for sub-sampling9 with sampling patterns rotated from frame to frame to form the fully sampled k-space center for coil sensitivity estimation. Iterative reconstruction was individually applied to the velocity images and subsequently passed to phase difference reconstruction. CS 4D flow images were acquired on a clinical 1.5T scanner (MAGNETOM Aera, Siemens Healthcare, Erlangen, Germany) in 5 healthy volunteers with three effective acceleration rates (Reff) 5.7, 7.7 and 10.2 (TE/TR=2.3/4.6ms, temporal resolution=37.7ms, spatial resolution=2.5×2.5×2.5mm3, 24 slices, bandwidth=540Hz/pixel, FA=7°, VENC=150cm/s for all three velocity encoding directions, NAV acceptance window=8mm). For comparison, a conventional 4D flow with rate 2 GRAPPA acceleration was acquired with the equivalent spatial/temporal resolution and spatial coverage. In each volunteer, 4D flow images were acquired to cover the entire thoracic aorta and the proximal parts of the supra-aortic branches. Preprocessing was applied to correct for Maxwell terms, eddy current induced background phase offsets, velocity aliasing and noise. A 3D angiogram (MRA) was computed to segment the aortic volume (Mimics, Materialise, Leuven, Belgium). Data analysis further included 3D blood flow visualization based on 3D streamlines and flow quantification at defined anatomic locations. Nine 2D planes were manually placed orthogonal to the aorta for flow quantification with 1 at the aortic root, 2 at the ascending aorta (AAo), 1 at the arch and 5 at the descending aorta (DAo) (EnSight, CEI, Apex, NC, USA). Flow-time curves and net flow were calculated for each analysis plane.Results
Figure 1 shows the respiratory position map of the k-space for one cardiac phase with CS Reff = 7.7 and ReCAR.. Table 1 summarizes the average acquisition time of 4D flow with GRAPPA R2 and CS. CS significantly reduced the total acquisition time (p<0.005). The CS 4D flow images were reconstructed on the scanner in less than 5 minutes using a GPU (Tesla K10, NVIDIA). Representative 4D flow magnitude images in the systolic phase and 3D MRA maximum-intensity-projections (MIPs) in sagittal orientation are shown in Figure 2, without significant blurring or artifacts noticed in CS 4D flow images. Figure 3 illustrates 3D streamlines for the systolic phase in 2 volunteers from four different 4D flow acquisitions. The mean flow curves from all five volunteers at root, arch and DAo5 are plotted in Figure 4a. CS Reff 10.2 tends to underestimate peak flow in DAo5 by 16.5%, while the flow patterns from CS Reff 5.7 and 7.7 match well with the reference. Regression analysis shows that there is strong correlation between net flow and peak flow when comparing CS 4D flow with conventional GRAPPA accelerated 4D flow MRI (p<0.005, Figure 4b,c).Conclusion
The long acquisition time of conventional 4D flow with parallel acceleration limits its clinical applications. Highly accelerated 4D flow with CS and ReCAR acquisition enables a 3-minute aortic protocol with isotropic 2.5mm voxels and fast inline reconstruction, making it feasible to be integrated into clinical workflow. Initial volunteer tests show a good match of flow quantification results with the conventional technique.Acknowledgements
No acknowledgement found.References
- Markl M, Kilner P, Ebbers T. Comprehensive 4D velocity mapping of the heart and great vessels by cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2011; 13:7.
- Schnell S, Markl M, Entezari P, Mahadewia RJ, Semaan E, Stankovic Z, Collins J, Carr J, Jung B. k-t GRAPPA accelerated four-dimensional flow MRI in the aorta: effect on scan time, imagequality, and quantification of flow and wall shear stress. Magn Reson Med. 2014 Aug;72(2):522-33.
- Kim D, Dyvorne HA, Otazo R, Feng L, Sodickson DK, Lee VS. Accelerated phase-contrast cine MRI using k-t SPARSE-SENSE. Magn. Reson. Med. 2012; 67:1054-1064.
- Kwak Y, Nam S, Ak_cakaya M, Basha TA, Goddu B, Manning WJ, Tarokh V, Nezafat R. Accelerated aortic ow assessment with compressed sensing with and without use of the sparsity of the complex diverence image. Magn. Reson. Med. 2013; 70:851-858.
- Knobloch V, Boesiger P, Kozerke S. Sparsity transform k-t principal component analysis for accelerating cine three-dimensional ow measurements. Magn. Reson. Med. 2013; 70:53-63.
- Dyvorne H, Knight-Greenfield A, Jajamovich G, Besa C, Cui Y, Stalder A, Markl M, Taouli B. Abdominal 4D Flow MR Imaging in a Breath Hold: Combination of Spiral Sampling and Dynamic Compressed Sensing for Highly Accelerated Acquisition. Radiology. 2015;275:245-254.
- Markl M, Harloff A, Bley TA, et al. Time-resolved 3D MR velocity mapping at 3T: improved navigator-gated assessment of vascular anatomy and blood flow. J Magn Reson Imaging 2007;25:824–831.
- Bailes DR, Gilderdale DJ, Bydder GM. Respiratory ordered phase encoding (ROPE): a method for reducing respiratory motion artifacts in MR imaging. J Comput Assist Tomogr 1985;9:835–838.
- Forman C. et al.; High-Resolution 3D Whole-Heart Coronary MRA: A Study on the Combination of Data Acquisition in Multiple Breath-Holds and 1D Residual Respiratory Motion Compensation; Magn Reson Mater Phy; doi: 10.1007/s10334-013-0428-x (2014).
Figures

Table 1.
Total acquisition time of 4D flow data with GRAPPA R2 and CS.
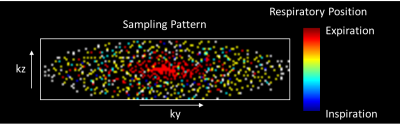
Figure 1. The respiratory position
map of k-space for one cardiac phase with CS Reff = 7.7 and ReCAR.
The kz-ky sampling pattern was designed to achieve a variable density spiral
phyllotaxis pattern for CS while minimizing respiration induced artifacts(blurring ghosting) by acquiring central k-space data during end-expiration and outer k-space during inspiration.
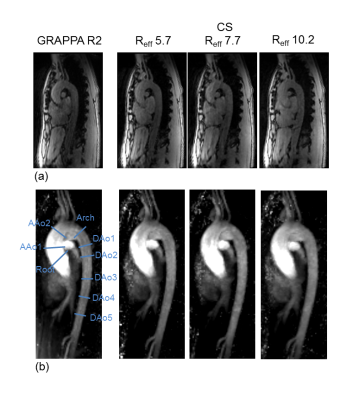
Figure 2.
Example of 4D flow data in one volunteer: (a) magnitude images in systolic
phase. (b) 3D sagittal MRA MIPs using the conventional 4D flow with GRAPPA R2
and CS 4D flow with Reff = 5.7, 7.7 and 10.2
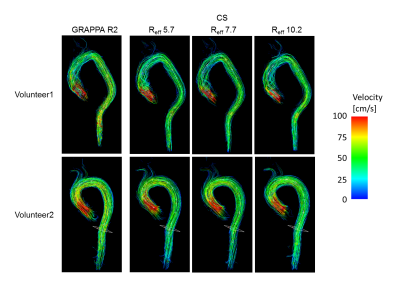
Figure 3. Examples of streamlines in the systolic phase in two volunteers using the conventional 4D flow with
GRAPPA R2 and CS 4D flow with Reff = 5.7, 7.7 and 10.2.
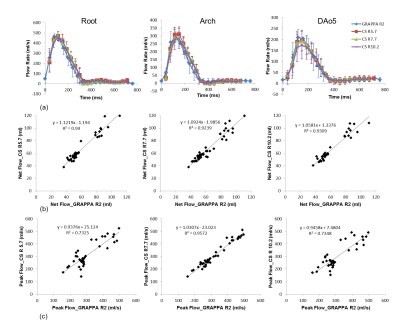
Figure 4. (a) The
mean flow curves from all five volunteers at root, arch and DAo5. (b) The
correlation plots of net flow between the CS 4D flow and the reference. (c) The
correlation plots of peak flow between the CS 4D flow and the reference.