0662
Brainstem abnormalities in structural MRI of young children with Autism Spectrum Disorder: evaluation of inter-method agreement.1INFN, Pisa, Italy, 2Humanities and Social Science, University of Strathclyde, Glasgow, United Kingdom, 3IRCCS Stella Maris, Pisa, Italy
Synopsis
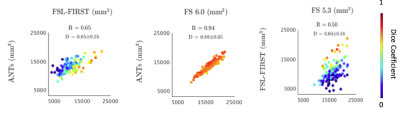
Structural MRI studies have pointed out the potential role of the brainstem in the pathophysiology of ASD. However, the findings in volume alterations in subjects with ASD are controversial. In this study, structural MRI was used to measure brainstem volume in a group of 152 young children with and without ASD, with five different methods (FSL-FIRST, ANTs, FS 5.3, FS 6.0, FS 6.0 with substructures). One out of five (FSL-FIRST) showed poor agreement with the other segmentation methods, which, by contrasts, consistently showed Pearson correlations greater than 0.93 and average Dice indexes greater than 0.76 in comparison among each other.
Introduction
Deficit in social communication abilities and the presence of restricted, repetitive behaviours represent the core features of autism spectrum disorder (ASD) 1. In addition sensorimotor abnormalities have been consistently reported in ASD individuals 2 as an early impairment 3 that may precede the development of ASD distinctive characteristics 4. Motor abilities depend on multiple interacting pathways including many connections that reach spinal motor neurons through the brainstem 5. Structural MRI studies of ASD individuals have pointed out the potential role of the brainstem in the pathophysiology of ASD 6,7. However, the findings in volume alterations in subjects with ASD with respect to matched controls are controversial both in adults and children cohorts with some early studies that did not detect any significant differences between the ASD and control samples 8,9,10,11,12. For this reason, it is important to investigate the contribution to variability of brainstem volume measurements performed with different automated methods.
Methods
Structural MRI was used to measure both volume and shape of the brainstem in a group of 76 subjects with ASD, including 38 males [mean age ± SD = 53 ± 16; age range = 27-87 months] and 38 females [mean age ± SD = 53 ± 18; age range = 25-88 months] and in a group of 76 control subjects matched by age, gender and non-verbal-IQ (NVIQ). All MRI scans were acquired in the same tertiary care hospital (IRCCS Stella Maris Foundation, Pisa, Italy) using a 1.5 T MR Neuro-optimized System (GE HealthCare, USA) fitted with 40 mT/m high-speed gradients. The standard MR protocol for children included a whole brain Fast Spoiled Gradient Recalled acquisition in the steady-state T1-weighted series (FSPGR). Isotropic images were collected in the axial plane with repetition time 12.4 ms, echo time 2.4 ms, inversion time 700 ms, flip angle of 10, yielding 124 contiguous 1.1 mm slices with an in-plane resolution of 1.1x1.1 mm2. Volume segmentations were carried out through some largely-used neuroimaging analysis tools for brain structure segmentation: FIRST 13 (FSL version 5.0.8), FreeSurfer 14 (version 5.3, 6.0 and 6.0 with brainstem substructure extraction) and ANTs (Advanced Normalization Tools) 15. In addition, we performed a shape analysis of the brainstem segmentations by using a standard SPHARM-MAT procedure 16, which creates parametric surface models using spherical harmonics. For statistical models of the signal extracted on the surfaces, we used SurfStat 17, a free software tool which performs statistical analysis of univariate and multivariate surface and volumetric data using linear mixed effect models and random field theory. The inter-method agreement of brainstem segmentation was quantified in terms of Pearson correlations between pairs of volumes obtained by different methods, whereas the degree of overlap between segmented masks was evaluated in terms of the Dice index. In addition, a statistical examination of brainstem volume was performed using the analysis of variance (ANOVA) test. A comparison between ASD and control subjects was performed both in the male and female groups separately and in the entire dataset using gender as covariate and considering subjects with or without by intellectual disability separatelyResults
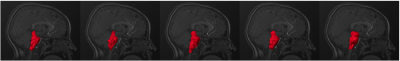
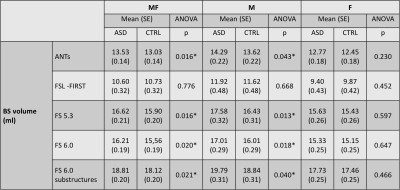
The 3D rendering of brainstem segmentations obtained with the 5 different methods (ANTs, FSL-FIRST, Freesurfer 5.3, 6.0, and 6.0 with substructures, respectively) for a sample subject are shown in figure 1. The renderings are overlaid onto the corresponding median sagittal view of the T1-weighted MRI. Three examples of comparison between methods in terms of scatter plots of brainstem volumes provided by the algorithms under investigations are reported in figure 2. Alongside the scatter plots, the Pearson correlation coefficients (R) are reported to summarize the overall agreement in terms of volumetric information. Both in terms of the Pearson correlation and of the Dice index, FSL-FIRST showed poor agreement with the other segmentation methods, which, by contrasts, consistently showed Pearson correlations greater than 0.93 and average Dice indexes greater than 0.76 in comparison among each other. The shape analysis confirmed the discrepancies among different segmentation methods, with particular reference to the FSL-FIRST under- and over-segmentation problems in specific brainstem regions. The BS volume resulted significantly higher in ASD when compared to controls both in the entire sample and in male subgroup (Figure 3) for ANTs, FS 5.3, and FS 6.0 computations. No statistically significant volume differences were obtained for females in BS total volume.Conclusions
This study suggests that research on brainstem alterations should cross-validate findings across multiple methods. Nevertheless, we reliably detected an enlargement of brainstem volume in the whole sample and in the male cohort.Acknowledgements
This work has been supported by the Tuscany Government (PAR-FAS 2007-2013, Bando FAS Salute 2014) through the ARIANNA Project (C52I16000020002.References
1. American Psychiatric Association, T. F. on D.-I. Diagnostic and statistical manual of mental disorders: DSM-IV. (2013).
2. Fournier, K. A., Hass, C. J., Naik, S. K., Lodha, N. & Cauraugh, J. H. Motor Coordination in Autism Spectrum Disorders: A Synthesis and Meta-Analysis. J. Autism Dev. Disord. 40, 1227–1240 (2010).
3. Teitelbaum, P., Teitelbaum, O., Nye, J., Fryman, J. & Maurer, R. G. Movement analysis in infancy may be useful for early diagnosis of autism. Proc. Natl. Acad. Sci. U. S. A. 95, 13982–7 (1998).
4. Ozonoff, S., Heung, K., Byrd, R., Hansen, R. & Hertz-Picciotto, I. The onset of autism: patterns of symptom emergence in the first years of life. Autism Res. 1, 320–8 (2008).
5. Drew, T., Prentice, S. & Schepens, B. in 251–261 (2004). doi:10.1016/S0079-6123(03)43025-2
6. Gaffney, G. R., Kuperman, S., Tsai, L. Y. & Minchin, S. Morphological evidence for brainstem involvement in infantile autism. Biol. Psychiatry 24, 578–586 (1988).
7. Jou, R. J., Frazier, T. W., Keshavan, M. S., Minshew, N. J. & Hardan, A. Y. A two-year longitudinal pilot MRI study of the brainstem in autism. Behav. Brain Res. 251, 163–167 (2013).
8. Elia, M. et al. Clinical Correlates of Brain Morphometric Features of Subjects With Low-Functioning Autistic Disorder. J. Child Neurol. 15, 504–508 (2000).
9. HARDAN, A. Y., MINSHEW, N. J., HARENSKI, K. & KESHAVAN, M. S. Posterior Fossa Magnetic Resonance Imaging in Autism. J. Am. Acad. Child Adolesc. Psychiatry 40, 666–672 (2001).
10. Herbert, M. R. et al. Dissociations of cerebral cortex, subcortical and cerebral white matter volumes in autistic boys. Brain 126, 1182–92 (2003).
11. Hsu, M., Yeung-Courchesne, R., Courchesne, E. & Press, G. A. Absence of magnetic resonance imaging evidence of pontine abnormality in infantile autism. Arch. Neurol. 48, 1160–3 (1991).
12. Kleiman, M. D., Neff, S. & Rosman, N. P. The brain in infantile autism: are posterior fossa structures abnormal? Neurology 42, 753–60 (1992).
13. Patenaude, B., Smith, S. M., Kennedy, D. N. & Jenkinson, M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage 56, 907–922 (2011).
14. Fischl, B. et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33, 341–355 (2002).
15. Avants, B. B., Epstein, C. L., Grossman, M. & Gee, J. C. Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 12, 26–41 (2008).
16. Brechbühler, C., Gerig, G. & Kübler, O. Parametrization of Closed Surfaces for 3-D Shape Description. Comput. Vis. Image Underst. 61, 154–170 (1995).
17. Worsley, K. et al. SurfStat: A Matlab toolbox for the statistical analysis of univariate and multivariate surface and volumetric data using linear mixed effects models and random field
Figures