0652
MRI-guided robotic arm (MgRA) drives optogenetic activation of the rat corpus callosum1Research Group of Translational Neuroimaging and Neural Control, High-Field Magnetic Resonance, Max Planck Institute for Biological Cybernetics, Tuebingen, Germany, 2Graduate Training Centre of Neuroscience, University of Tuebingen, Tuebingen, Germany, 3The Werner Reichardt Centre for Integrative Neuroscience, University of Tuebingen, Tuebingen, Germany
Synopsis
An MRI-compatible robotic arm was developed to provide a precise fiber positioning for optogenetic fMRI of the rat brain. Corpus callosum connects two hemispheres through a thin sheet of spreading fiber bundle with only a few hundred micron thickness in the brain. This work shows that MgRA can guide fiber optic to precisely target the callosal fiber bundles. The optogenetically driven callosal axonal fiber-mediated neural activity leads to strong antidromic activation in the hemisphere with callosal neurons expressing ChR2 by fMRI.
Target Audience
Scientists who are interested in small animal fMRI with cutting-edge techniques.Introduction
Functional changes across brain hemispheres have been reported after unilateral cortical or peripheral nerve injury1, 2, 3. Interhemispheric callosal connections usually underlie this cortico-cortical plasticity3, 4. By targeting the corpus callosal (CC) fiber expressing Channelrodopsin5, we could study the callosum mediated brain plasticity optogenetically. However, the thickness of the callosal fibers along the dorsal-ventral axis is only a few hundred micron in rats6. It is difficult to precisely target the callosal fibers by brain atlas-based conventional stereotaxic positioning. Here, we developed an MRI-guided robotic arm (MgRA) positioning system to target callosal fibers with high precision inside a 14.1T MR scanner and mapped the BOLD-fMRI functional patterns driven by optogenetic activation of the callosal fibers.Methods
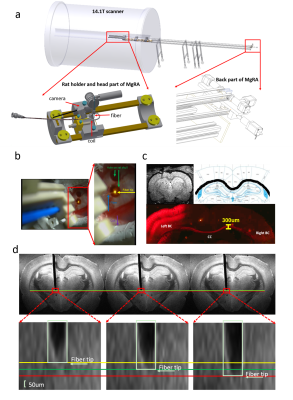
An MRI-compatible, automatic robotic control multi-degree robotic arm has been developed in 14.1T scanner, as shown in Fig.1a. Fig.1b illustrates the camera-based signals for experiment setup in the iso-center of the horizontal bore magnet. Alpha chloralose anesthetized rat is fixed by ear bars and tooth bar on the custom-designed MRI-compatible rat holder. A 24mm-diameter custom-designed transmit/receive surface coil was located on the rat brain while the fiber could be inserted into the brain to target CC through the hole of the coil driven by the robotic arm. MRI scans were performed using 2D rapid acquisition with relaxation enhancement (RARE) sequence: TR, 1200ms, TE, 7.5ms, 1.92cmX1.68cm FOV, 128X112 matrix, 0.15X0.15X0.6 mm3 spatial resolution. AAV5.CAG.ChR2.mcherry viral vectors were stereotaxically injected in the left S1FL (primary somatosensory cortex, forelimb region) in 4-week old rats. The detailed surgical procedures for optogenetic fMRI were described previously7. The optical fiber(~200um) delivered blue light pulses (473nm) at 5Hz, 20ms width with 15s duration for the fMRI block design. fMRI scans were performed using 3D Echo planar imaging sequence: TR, 1.5s, TE,11.5ms, 2.24X1.92X1.92cm3 FOV, 56X48X48 matrix, 400X400X400 um3 spatial resolution. MRI data analysis was performed using Analysis of Functional NeuroImages software(NIH, Bethesda).Results
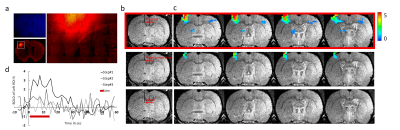
Fig.1 illustrates that MgRA could drive fiber optic along the dorsal-ventral axis every 50 µm step, of which the accuracy is sufficient to guide the fiber optic to target CC. Channelorhodopsin-2 (ChR-2) was expressed in the callosal projection neurons in left hemisphere so that only the axonal fibers to the opposite direction was labeled(Fig. 2a). Fig.2b shows three locations of the fiber optic along the predefined trajectory—touching (step#1), nearly penetrating (step#2), and bypass (step#3) CC. Upon the optogenetic stimulation of the CC of step#1, there was significant increase of the fMRI signals of anti-dromic neuron activity from the axonal fibers backward to the soma of callosal projection neurons in the ipsilateral barrel cortex, as well as a small projection-induced BOLD signal in the contralateral hemisphere (Fig 2d). When the fiber optic was nearly penetrating or bypass the CC, less and no BOLD signals were detected, respectively. Fig. 2c illustrates the time courses from the anti-dromic activity-induced BOLD signals in the ipsilateral hemisphere.Conclusion
The optogenetic activation of callosal axonal fibers led to strong antidromic activity in the hemisphere where the ChR2 expressing callosal neurons were located. The lack of fMRI signal in the contralateral hemisphere indicates that the net callosal inputs to the local excitatory-inhibitory circuits may not lead to sufficient BOLD signal detected by fMRI. MgRA provides sufficient targeting accuracy and flexibility for brain intervention for the multi-modal fMRI platform.Acknowledgements
We thank Mr. Shanyi Yu for building up the first prototype of the robotic arm and Mr. Johannes Boldt for helping to improve the MgRA system. This work is supported by the Max-Planck-Society and the China Scholarship Council (PhD fellowship to Yi Chen).References
1. M. E. Magnuson, G. J. Thompson, W. J. Pan, and S. D. Keilholz, 'Effects of Severing the Corpus Callosum on Electrical and Bold Functional Connectivity and Spontaneous Dynamic Activity in the Rat Brain', Brain Connect, 4 (2014), 15-29.
2. G. Pelled, D. A. Bergstrom, P. L. Tierney, R. S. Conroy, K. H. Chuang, D. Yu, D. A. Leopold, J. R. Walters, and A. P. Koretsky, 'Ipsilateral Cortical Fmri Responses after Peripheral Nerve Damage in Rats Reflect Increased Interneuron Activity', Proc Natl Acad Sci U S A, 106 (2009), 14114-9.
3. X. Yu, S. Chung, D. Y. Chen, S. Wang, S. J. Dodd, J. R. Walters, J. T. Isaac, and A. P. Koretsky, 'Thalamocortical Inputs Show Post-Critical-Period Plasticity', Neuron, 74 (2012), 731-42.
4. X. Yu, and A. P. Koretsky, 'Interhemispheric Plasticity Protects the Deafferented Somatosensory Cortex from Functional Takeover after Nerve Injury', Brain Connect, 4 (2014), 709-17.
5. X. Yu, 'When Photons Meet Protons: Optogenetics, Calcium Signal Detection, and Fmri in Small Animals', in Small Animal Imaging Basics and Practical Guide, ed. by F. Kiessling, B Pichler and P. Hauff (Springer International Publishing, 2017).
6. J. Zhou, Y. Wen, L. She, Y. N. Sui, L. Liu, L. J. Richards, and M. M. Poo, 'Axon Position within the Corpus Callosum Determines Contralateral Cortical Projection', Proc Natl Acad Sci U S A, 110 (2013), E2714-23.
7. X. Yu, Y. He, M. Wang, H. Merkle,
S. J. Dodd, A. C. Silva, and A. P. Koretsky, 'Sensory and Optogenetically
Driven Single-Vessel Fmri', Nat Methods,
13 (2016), 337-40.
Figures