0651
AN ACTIVE BIOPSY NEEDLE DESIGN FOR MRI GUIDED PROSTATE BIOPSY1Biomedical Engineering, Institute of Biomedical Engineering, Bogazici University, Istanbul, Turkey
Synopsis
In this work, we present a novel low profile and active biopsy needle for MRI guided prostate interventions. We show that using conductive ink printed non-planar RF antenna components does not compromise overall device profile while maintaining required mechanical properties for clinical grade devices. We confirmed that the biopsy needle design is conspicious under MRI while maintaining RF induced heating below the allowed limits. This work demonstrates the feasibility of developing ultra low profile active interventional devices and potentially enables physicians to perform MRI guided prostate interventions.
INTRODUCTION
Prostate cancer is the second frequent cancer among the cancer types while it is the most common malignant tumor in men in the USA and western Europe [1]. Transrectal ultrasound imaging (TRUS) guided percutaneous biopsy procedure is the golden standard for prostate cancer diagnosis. Recently, Magnetic Resonance Imaging (MRI) has been a strong candidate against TRUS to guide prostate interventions [2, 3]. MRI provides superior soft tissue contrast and has sufficient spatial resolution to identify small anatomical structures such as early stage tumors [4]. However, advances in MRI guided interventions remain incremental due to the lack of clinical grade MRI compatible interventional devices, i.e. biopsy needles. In this study, we aim to introduce a novel MRI compatible and low profile active biopsy needle prototype, fabricated using a new method previously introduced by our group [5], to maximize device conspicuity while maintaining mechanical properties of a conventional biopsy needle.METHODS
Active Needle Design:
An MRI compatible nitinol hypotube (Confluent, CA, USA) with 1 mm diameter was cut in 15 cm length to be used as the needle body. Needle tip and biopsy groove were shaped through laser cutting process (Lasag, Bern, Switzerland). Multiple RF receiver loop antennas including the transmission line were printed over the needle shaft using a custom design CNC based conductive ink printing system to reduce the overall needle profile. Bio-compatible heat shrink polymers (Vention Medical, CO, USA) were used as insulator layers. A conductive ink containing silver nano particles (Conductive Compounds, NH, USA) was used as the printing material. The total impedance of the loop RF antenna on the needle was matched to 50Ω at 63.66 MHz for 1.5T MRI system, with a conventional matching/tuning circuit using a Network Analyzer (4395A, Agilent, CA).
In Vitro Testing:
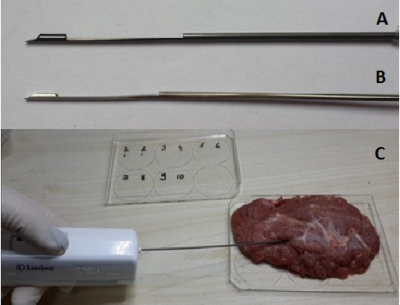
MRI visibility, RF induced heating and biopsy performances of the active prostate needle, were tested in vitro. MRI visibility performance of the active biopsy needle design was tested under 1.5 T MR system (Aera, Siemens, Erlangen, Germany) using water based phantom. GRE sequence (TE/TR =1.8/8.2 ms, FA = 45°, FOV = 300 mm, matrix = 192 x 192, slice thickness = 5 mm) was used during MRI scans. RF induced heating test was performed in ASTM 2182 gel phantom. Biopsy performance of the needle prototype was compared with a commercially available MRI conditional biopsy needle (PRIMECUT, TSK, Japan) through bench top tests using fresh veal (Figure 5). 10 biopsy samples were taken with each needle and tissue samples were weighted on a digital scale (Mettler-Toledo, OH, USA).
RESULTS
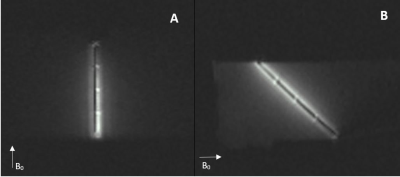
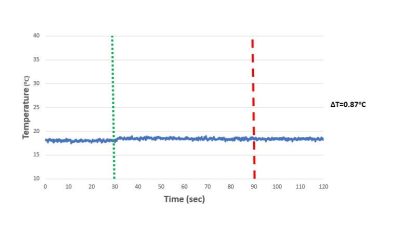
Identical coils were formed on each end of the biopsy groove for accurate needle localization during biopsy procedure. Each coil length was measured as 3.07 mm while the line width was 128 µm and the distance between lines was 166 µm (figure 1). Additional loop coils were formed to indicate the overall insertion length during the biopsy procedure (figure 2). Overall device profile for the final prototype was measured as 1.034 ± 0.001 mm. Active biopsy needle prototype was visualized distinguishably under MRI. Bright spots on needle images indicate the actual coil locations on needles (figure 3). During RF induced heating test, maximum temperature rise was recorded as 0.87 ºC at the tip of the active biopsy needle (figure 4). Biopsy samples taken with commercial needle were weighted as 0.00042 ± 0.00023g and samples taken with custom made needle were weighted as 0.0038 ± 0.0014g.
DISCUSSION AND CONLUSION
Active prostate biopsy needle prototypes were successfully fabricated using nitinol hypotubes, heat shrink polymer insulators and printed RF antenna parts formed using thin film printing system. The overall device profile increased only 0.034 ± 0.001mm. The smooth active device surface profile eliminates the potential risk of damaging anatomical structures during needle insertion which is a common problem for interventional MRI devices incorporating conventional rigid components. The biopsy needle prototypes were distinguishably conspicuous under MRI while maintaining the mechanical requirements without compromising the overall device profile. During the in vitro heating tests, RF induced temperature rise remained under clinically significant levels (<2 ᵒC) . Biopsy performance test of the active needle resulted almost 10 times better than the equivalent commercial biopsy needle. In this study, we have successfully shown the feasibility of low profile active prostate biopsy needle design dedicated to interventional MRI procedures using heat shrink polymers and conductive ink printing system. Using active device components embedded to heat shrink polymers would be an attractive technique to convert MRI conditional but not visible devices to MRI compatible devices.Acknowledgements
This research was supported by grants from TUBITAK project (115E271) to
Assist. Prof. Dr. Özgür Kocatürk
References
1. Siegel, R., et al., Cancer statistics, 2014. CA Cancer J Clin, 2014. 64(1): p. 9-29.
2. Yiallouras, C. and C. Damianou, Review of MRI positioning devices for guiding focused ultrasound systems. Int J Med Robot, 2015. 11(2): p. 247-55.
3. Yiallouras, C., N. Mylonas, and C. Damianou, MRI-compatible positioning device for guiding a focused ultrasound system for transrectal treatment of prostate cancer. Int J Comput Assist Radiol Surg, 2014. 9(4): p. 745-53.
4. Lederman, R.J., Cardiovascular interventional magnetic resonance imaging. Circulation, 2005. 112(19): p. 3009-17.
5. Yildirim, K.D., et al. A Novel method for developing clinical grade active devices dedicated to interventional MRI procedures. in ISMRM 2016. 2016.
Figures