0646
SMART tracking: SiMultaneous Anatomical imaging and Real-Time needle tracking1Image Sciences Institute, UMC Utrecht, Utrecht, Netherlands
Synopsis
We propose a passive needle tracking method for MR-guided interventions. We combined undersampled 2D radial multi-echo acquisitions, the white marker phenomenon, and simulations and template matching of artifacts around the needle tip to achieve real-time (10 Hz) tracking of the needle tip, while simultaneously being able to reconstruct anatomical images using a sliding window reconstruction. The method is demonstrated to smoothly track the insertion of a needle in porcine tissue while also showing deformations in the anatomical image caused by the needle insertion. These results bridge a gap between active and passive tracking of needles.
Introduction
MR-guidance during interventions has shown promise in a variety of applications, including needle biopsies, vascular interventions, and radiation therapy. The availability of active tracking catheters has caused real-time MR-guided vascular interventions to become a serious alternative for X-ray based interventions, particularly cardiac interventions. However, in needle directed interventions, incorporating active tracking hardware in a metallic needle can be challenging due to the size of the device and heating concerns (1). Passive tracking of metallic needles in MRI does not have these hardware requirements, but the tracking is slower and accuracy may be limited because the needle does not generate contrast at the exact location of the device and instead causes artifacts in the image (2). Previous work has shown that simulations of these artifacts can be used to localize metal devices in MRI (3). In this abstract we show that this methodology can be applied to undersampled radial multi-echo acquisitions to achieve real-time passive device tracking while simultaneously being able to reconstruct anatomical images with minimal dephasing artifacts, which bridges a gap between passive and active tracking of needles in MRI.Methods
Acquisition:
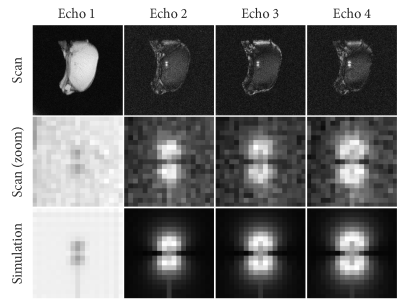
We acquired multi-echo radial 2D gradient echo images at 1.5T (Philips Achieva, Best, The Netherlands) with the following parameters: 1.2x1.2x15 mm resolution, 230x230x15 mm FOV, TE1/TE2/TE3/TE4/TR = 1.73/3.18/4.62/6.07/8.46 ms, flip angle 10°, number of radial profiles 192, dynamic scan time 1.6 seconds. A bit-reversed profile order (4) was used to allow undersampled reconstructions up to a factor of 16. A dephaser gradient in the slice direction was added after the first echo to induce the white marker phenomenon in the 2nd to 4th echoes, which creates positive contrast near metal devices and increases specificity (5).
Reconstruction:
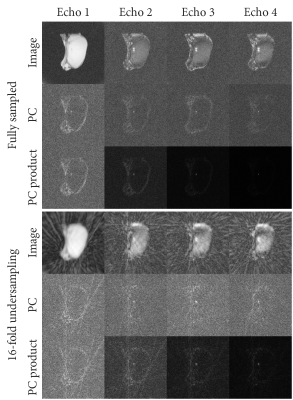
For each 16-fold undersampled frame we performed device localization using Phase Correlation (PC) template matching (6). Because PC is calculated in the frequency domain, this can be trivially applied to non-uniform and undersampled k-space data. For each of the echoes, we simulated templates of the tip of an MRI-compatible titanium needle (20G MRI Chiba, Somatex, Berlin, Germany), including the dephaser gradient in the 2nd to 4th echoes (Figure 1). After applying PC, images were reconstructed by gridding using the NUFFT library (7) after sampling density correction was applied to the radial profiles. The PC images for each echo were multiplied to create a single correlation image that contains the information from all echoes (Figure 2). Anatomical images were reconstructed from the first echo of the acquisition using a sliding window approach on the undersampled frames.
Tracking:
The tip of the needle was tracked by detecting a maximum in the combined PC image. Because the location of this maximum is affected by undersampling artifacts, a Kalman filter with a simple motion model (position and velocity) was applied to track the tip of the needle over time.
Experiments:
We applied the proposed method to two sequences of insertions of the titanium needle into ex vivo porcine tissue. The angle of insertion of the needle relative to B0 was 0 degrees and 45 degrees in these sequences.
Results
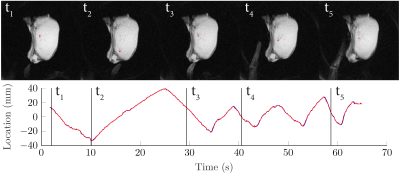
Figure 3 shows still frames and the location of the tracked needle tip during a number of insertions and retractions of the needle along B0. Figure 4 shows an animation of part of this tracked sequence. Figure 5 shows an animation of the insertion and retraction of the needle at an angle of 45 degrees relative to B0.Discussion & Conclusion
We have demonstrated that combining undersampled radial multi-echo acquisitions with template matching-based passive device localization enables real-time (10 Hz) localization of metal devices, while also being able to reconstruct non-accelerated anatomical images (0.6 Hz). Because the anatomical images are acquired at the shortest echo time, metal artifacts in these images are relatively benign.
The results show that the tracked tip of a titanium needle was stable and consistent with the linear insertion and retraction of the needle. While the accuracy of template matching based object localization has been demonstrated in previous work for stationary devices in unaccelerated scans (8), further experiments will be needed to quantitatively establish the accuracy on undersampled acquisitions with moving devices.
The passive tracking method proposed
in this abstract possesses some of the advantages that are traditionally only
available with active tracking methods: accurate tracking of devices at high
framerates, the ability to include real-time anatomical scanning, and the
capability of automatic slice positioning. Furthermore, the method is flexible
with respect to the type of device and acquisition parameters. No specialized
hardware is required, as long as the artifacts around the device can be
accurately simulated and provide enough information about the location of the
device.
Acknowledgements
This work is part of the research programme Applied and Engineering Sciences (TTW) with project number 10712 which is (partly) financed by the Netherlands Organization for Scientific Research (NWO).References
1. Saikus CE, Ratnayaka K, Barbash IM, Colyer JH, Kocaturk O, Faranesh AZ, Lederman RJ. MRI-guided vascular access with an active visualization needle. J. Magn. Reson. Imaging 2011;34:1159–1166.
2. Müller-Bierl B, Graf H, Lauer U, Steidle G, Schick F. Numerical modeling of needle tip artifacts in MR gradient echo imaging. Med. Phys. 2004;31:579–587.
3. Zijlstra F, Bouwman JG, Braškutė I, Viergever MA, Seevinck PR. Fast
Fourier-based simulation of off-resonance artifacts in steady-state gradient
echo MRI applied to metal object localization. Magn. Reson. Med.
2017;78:2035–2041.
4. Chan RW, Ramsay EA, Cheung EY, Plewes DB. The influence of radial undersampling schemes on compressed sensing reconstruction in breast MRI. Magn. Reson. Med. 2012;67:363–377. doi: 10.1002/mrm.23008.
5. Seppenwoolde J-H, Viergever MA, Bakker CJG. Passive tracking exploiting local signal conservation: The white marker phenomenon. Magn. Reson. Med. 2003;50:784–790.
6. Kuglin CD, Hines DC. The Phase Correlation Image Alignment Method. In: Proc. of the IEEE Int. Conf. on Cybernetics and Society. ; 1975. pp. 163–165.
7. Fessler JA, Sutton BP. Nonuniform fast Fourier transforms using min-max interpolation. IEEE Trans. Signal Process. 2003;51:560–574
8. Zijlstra F, Moerland MA, van der Voort van Zyp JRN, Noteboom JL, Viergever MA, Seevinck PR. Challenges in MR-only seed localization for postimplant dosimetry in permanent prostate brachytherapy. Med. Phys. 2017;44:5051–5060.
Figures