0638
Reducing Radiofrequency-induced Heating in Realistic Deep Brain Stimulation Lead Trajectories using Parallel TransmissionPei-Shan Wei1, Benson Yang1, Clare E. McElcheran2, Laleh Golestanirad3, and Simon J. Graham1,4
1Physical Sciences, Sunnybrook Research Institue, Toronto, ON, Canada, 2Baylis Medical, Mississauga, ON, Canada, 3Massachusetts General Hospital, Boston, MA, United States, 4Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada
Synopsis
Patients with deep brain stimulation devices have a safety concern for localized heating that can arise during MRI. Clinically, the lead trajectory for the surgical stimulation procedure varies among patient population. We have shown in previous numerical simulations that the lead trajectory would affect the performance of heating suppression in patient-specific pTx optimization. The present work experimentally validated that RF-induced heating can be suppressed for a high-risk patient-specific lead trajectory, using optimal pTx input parameters.
Introduction
A major MRI safety concern for patients with deep brain stimulation (DBS) devices is the potential for localized heating that can arise if the leads couple substantially with the excitatory radiofrequency (RF) field. It has been shown that parallel RF transmission (pTx) technology1 may be very useful to reduce such RF coupling and localized power deposition3. Clinically, the lead trajectory can be considerably different between patients as the target of stimulation and the surgical implementation2 are customized on an individual basis. We have previously used numerical simulations to demonstrate that the lead trajectory is an important factor for patient-specific pTx optimization to suppress heating3. In the present work, we conduct a proof-of-concept validation experiment to demonstrate that RF-induced heating can be suppressed for a high-risk patient-specific lead trajectory, using a set of optimized pTx input parameters.Methods
From numerical simulation data that estimated the localized heating effects for the real lead trajectories of nine DBS patients4, the trajectory with worst-case heating was selected for experimental work (Fig. 1). An insulated 61 cm copper wire with an exposed tip was placed in a uniform head phantom to approximate this lead trajectory (Fig. 2A), including a looped four-turn figure-eight pattern representative of how excess lead length was bundled at the skull by the neurosurgeon. The penetrating portion of the lead conformed closely to the specific patient model. The head phantom (Fig. 2B) was filled with a 4.5 L oil-in-gelatin mixture5 (oil = 3 %, NaCl = 4.5 g/L, and gelatin = 170 g/L) to mimic gray matter tissue and its corresponding electromagnetic properties. Subsequent RF heating experiments were conducted on a 3 T MRI system (MAGNETOM Prisma, Siemens, Germany). A single-channel RF excitation pulse from the MRI system was split into a custom 4-channel pTx system and transceiver coil to suppress heating using optimized RF shim settings. The amplitude (A) and phase (Φ) of the pulse on each channel was controlled by a commercial RF modulator (CGP-128-4C, CPC, Hauppauge, USA). Amplitude and phase settings for “suppression mode” were determined in simulation as in our previous work4, using an optimization algorithm to minimize local SAR in a cubic (0.5 cm3) region of minimization (ROM) surrounding the tip of the lead, while maximizing RF magnetic field uniformity over a large field of view. The suppression mode settings were A = 1.24, 0.5, 1.25, and 0.71, Φ= 62°, 347°, 290°, and 310°. For comparison, pTx MRI was also performed in “quadrature mode” with identical RF pulse amplitude for each channel and a phase shift of 90° between neighbouring channels. Imaging was performed with a turbo spin-echo sequence with high power deposition (acquisition matrix = 256 x 205, in-plane resolution = 0.5 mm x 0.5 mm, TR/ TE = 516 ms / 6.7 ms, flip angle = 150°, slice thickness = 2 mm, and acquisition time = 3.8 mins). During imaging, the temperature change at the lead tip was monitored with a fibre-optic temperature probe (OTG-MPK5, Opsens Inc., Canada).Results
Images in the plane of the lead tip for pTx quadrature mode and suppression mode are shown in Fig. 3A and Fig. 3B, respectively. In the rectangular region (dashed lines) encompassing the ROM, suppression mode resulted in better image uniformity than quadrature mode with associated improvement in signal-to-noise ratio of 51 %. The time-dependent temperature changes produced by pTx MRI are shown for both modes in Fig. 4. During pTx quadrature mode, the temperature increased by 2.6 °C and did not reach steady state over a 4 min. time period, whereas suppression mode limited the temperature increase to <1 °C (62 % suppression) with good stability over approximately the last half of the image acquisition period.Discussion
Promising proof-of-concept results were obtained, demonstrating that suppression mode pTx can limit temperature increases to <1 °C at the lead tip for a high-risk, clinically realistic DBS lead trajectory, with locally-improved image uniformity compared to quadrature mode pTx. However, residual coupling effects were still observable in suppression mode pTx MR images, and even better image uniformity would be useful over a larger field of view. Some improvement is likely by accounting for hardware and experiment discrepancies in relation to parameters used in the numerical simulation procedures to determine the RF shim settings for suppression mode.Conclusion
Suppression mode pTx is capable of suppressing localized heating effects in implanted wires that are representative of high-risk realistic DBS lead trajectories. Further work will be required to improve image uniformity and to develop practical procedures for implementing pTx suppression modes when the electromagnetic properties of phantoms and tissues are not known in advance.Acknowledgements
The authors thank Mitacs, Siemens Healthcare and the Canadian Foundation for Innovation for funding support.References
- Katscher U, et al. Transmit SENSE. Magn Reson Med 2003; 49(1):144-50.
- Mattei E, et al. Complexity of MRI induced heating on metallic leads: experimental measurements of 374 configurations. Biomed Eng online 2008; 7(1):11.
- McElcheran CE, et al. Investigation of Parallel Radiofrequency Transmission for the Reduction of Heating in Long conductive Leads in 3 Tesla Magnetic Resonance Imaging. PLoS One 2015; 10(8):e0134379.
- McElcheran CE, et al. Parallel Transmission for Heating Reduction in Realistic Deep Brain Stimulation Lead Trajectories. ISMRM (2017)
- Yuan Y, et al. A heterogeneous human tissue mimicking phantom for RF heating and MRI thermal monitoring verification. Phys Med Biol 2012; 57(7): 2021-2037.
Figures
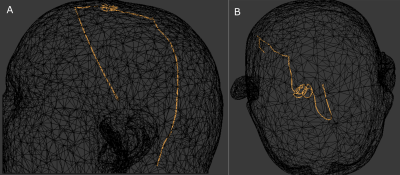
Figure 1: (A) sagittal
and (B) axial projections of the worst-case lead trajectory for RF coupling and
localized heating, from among nine DBS patients.
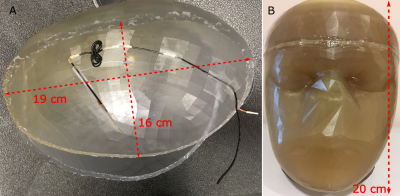
Figure 2: Photograph
showing (A) approximation of the worst-case lead trajectory in experiment; (B) implanted
wire within sealed uniform head phantom.
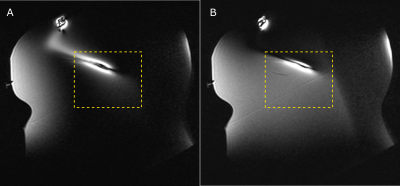
Figure 3: MR images in
the plane of the implanted wire using (A) quadrature mode pTx and (B)
suppression mode pTx.
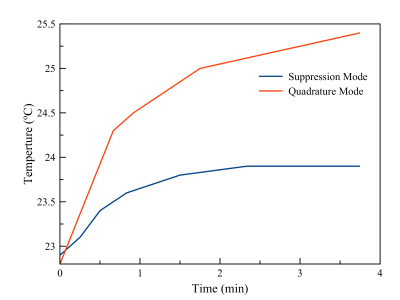
Figure 4: Temperature
as a function of time at the wire tip for quadrature mode pTx and suppression
mode pTx.