0633
Magnetic Resonance Angiography Reveals Increased Arterial Blood Supply and Tumorigenesis Following High Fat Feeding in a Mouse Model of Triple-negative Breast Cancer1Radiology, The University of Chicago, Chicago, IL, United States, 2Medicine, Section of Adult and Pediatric Endocrinology, Diabetes and Metabolism, The University of Chicago, Chicago, IL, United States, 3Pathology, The University of Chicago, Chicago, IL, United States, 4Medicine, the Section of Hematology and Oncology, The University of Chicago, Chicago, IL, United States
Synopsis
Breast cancer is the most commonly diagnosed malignancy among women in the US. Epidemiology shows that a high animal fat diet increases risk of triple-negative breast cancer (TNBC). Our previous work examined the effect of pre-pubertal exposure to high dietary animal fat in the SV40Tag mouse model of TNBC. We showed that a high animal fat diet changes mammary fat composition and increases incidence and aggressiveness of mammary cancers in this model. Here, we demonstrate using MR angiography that changes in fat composition and cancer incidence are paralleled by increases in vascular density in the mammary gland.
PURPOSE
High animal fat consumption is associated with increased incidence of triple negative breast cancer (TNBC).1,2 Our previous magnetic resonance imaging (MRI) studies demonstrated the effects of pre-pubertal exposure to high dietary fat in this model of TNBC,3 and the feasibility of detecting very early non-palpable intraductal mammary cancers in SV40Tag mice.4 The present study used time-of-flight (TOF) MR angiography to measure arterial blood volume feeding mammary glands in mice fed a low fat diet (LFD) or a high animal fat diet (HAFD).METHODS
Virgin female C3(1)SV40TAg mice (n=8) were weaned at 4 weeks old and fed a LFD (n=4, 3.7 kcal/g; 17.2% kcal from vegetable oil) or HAFD (n=4, 5.3 kcal/g; 60% kcal from lard). After 4 weeks on diet, in vivo MR images were acquired weekly from age 8-12 weeks. Fast spin echo MR images (RARE, TR/TEeffective=4000/20 ms, slice thickness=0.5 mm, in-plane resolution=0.1 mm) of inguinal mammary glands were acquired at 9.4 Tesla. TOF, a 2D flow compensated, gradient echo sequence with a short TR (TR/TEeffective=10/3 ms), was used to maximize inflow effects, depicting flowing blood as a bright signal; other parameters were as in RARE. Mice were sacrificed following in vivo serial MRI studies; inguinal mammary glands were excised and fixed for histology. Tumor and blood volumes were measured from manually traced ROI’s. Each invasive cancer was segmented and labeled. Volume of each tumor was calculated at five time points. Additional ROI’s on 2 slices of muscle were used to normalize signal intensity and reduce extraneous signal when calculating blood volume. Histopathology-blinded observers used MRI to classify tumors as invasive. Student’s t-Tests were performed for statistical analysis; p-value <0.05 was considered significant.RESULTS and DISCUSSION
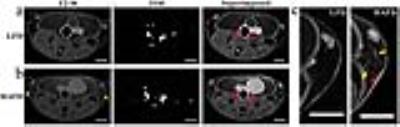
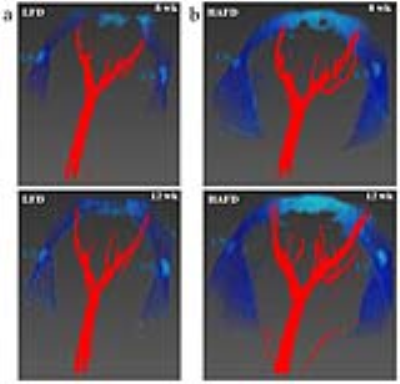
Mice fed HAFD (average body weight=19.75±2.02 g) did not gain significantly more weight than LFD fed mice (average body weight=18.20±1.04 g), p<0.075). Based on the size of invasive cancer (>400 microns in largest diameter) and signal intensities on T2-weighted MR images of 2.3 times that of muscle, invasive cancers were accurately identified using in vivo MRI.4 Figure 1 illustrates irregular, dilated ducts and increased blood supply in a HAFD mouse. The qualitative relationship is visualized more easily with a three-dimensional rendering seen in Figure 2. At week 12, with increased tumorigenesis following high fat feeding, average blood volume in mammary glands for HAFD-fed mice (2.56±0.27 μL) was 7-fold higher, compared to LFD-fed mice (0.36±0.10 μL), p<0.0001. Figures 3 and 4 illustrate the effects of HAFD on blood volume and mouse mammary cancer. Figure 3 shows the initial tumor volume (3a) and total tumor volume (3b). Figure 4 shows mammary gland blood volume (4a) and total blood volume (4b) of 8-12 week old mice on LFD and HAFD. Differences in tumor incidence and volume were significant at earlier time points (8-10 weeks) between HAFD and LFD mice (Figure 3a). Importantly, results in Figures 3b and 4a demonstrate a direct correlation between the total tumor volume and blood volume in the mammary gland. Tumor growth rates were 2-fold higher in HAFD mice (0.42±0.14 week-1) compared to LFD mice (0.21±0.03 week-1), with p<0.004, while the rate of increase in mammary gland blood volume was 2.2-fold higher in HAFD mice (0.29±0.11 week-1) compared to LFD mice (0.13±0.06 week-1), p<0.02. These results demonstrate the effectiveness of MR angiography for measurements of changes in vasculature as cancer develops. Histological images of mammary glands from HAFD mice showed more irregular, enlarged ducts, dilated blood vessels, and increased tumor invasiveness compared to the LFD group, consistent with MR images. Increased recruitment of arteries to cancerous mammary glands is probably associated with neo-angiogenesis, since increased arterial supply is required to feed dense, leaky capillaries.CONCLUSIONS
To the best of our knowledge, this study is the first to demonstrate a strong correlation between tumor and blood volume in mammary cancer in vivo, completely non-invasively without using contrast agents. These results further demonstrate the role of dietary fats on mammary cancers. TOF angiography can be used for serial studies of mammary gland/tumor vasculature in mouse models where repeat placement of I.V. lines is challenging. Additionally, analogous methods could be tested in humans to evaluate vasculature of suspicious lesions without using contrast agents.Acknowledgements
This research is supported by grants from the National Institutes of Health (R01-CA133490, R01-CA167785, and P30CA014599), Florsheim Foundation, Segal Foundation, and VPH prism grant from the European Union.References
1. Yang XR, Chang-Claude J, Goode EL, et al. Associations of breast cancer risk factors with tumor subtypes: a pooled analysis from the Breast Cancer Association Consortium studies. J Natl Cancer Inst. 2011;103(3):250-263.
2. Agurs-Collins T, Dunn BK, Browne D, Johnson KA, Lubet R. Epidemiology of health disparities in relation to the biology of estrogen receptor-negative breast cancer. Semin Oncol. 2010;37(4):384-401.
3. Mustafi D, Fernandez S, Markiewicz E, Fan X, Zamora M, Mueller J, Brady MJ, Conzen SD, Karczmar GS. MRI reveals increased tumorigenesis following high fat feeding in a mouse model of triple-negative breast cancer. NMR Biomed. 2017;30:e3758. https://doi.org/10.1002/nbm.3758.
4. Mustafi D, Zamora M, Fan X, Markiewicz E, Mueller J, Conzen SD, Karczmar GS. MRI accurately identifies early murine mammary cancers and reliably differentiates between in situ and invasive cancer: correlation of MRI with histology. NMR Biomed. 2015;28(9):1078-1086.
Figures