0630
Pre-existing inflammation in the brain promotes metastases invasion1Université de Sherbrooke, Sherbrooke, QC, Canada
Synopsis
We studied the effect of pre-existing inflammation on the genesis of brain metastases using MRI. Molecular MRI enhanced with VCAM-1 targeted microparticles of iron oxide enable the assessment of pre-existing vascular inflammation and then of metastasis implantation. The occurrence of brain metastases was increased by 2 fold when inflammation was present; this could be blocked with VCAM-1 antibody. This underlies the key role of VCAM-1 in tumor seeding to the brain, and suggests that preventive inflammation targeted treatment in cancer patients may minimize risks of brain metastasis.
Introduction
Brain metastases are the most frequently occurring intracranial malignant tumors and are associated with a particularly high morbidity and mortality. Inflammation is a critical component of cancer progression. Tumor-promoting inflammation facilitates the acquisition of malignant phenotype1. Studies in this field have mostly focused on how inflammation in the primary tumor microenvironment contributes to proliferation, invasion, malignant transformation and migration of tumor cells. While some light has been shed on the role of pre-existing inflammation in tumor development in non-central nervous system (CNS) secondary sites, little is known about its effect on metastases formation in the brain. Cell adhesion molecules (CAMs) expressed during the inflammatory process have been shown to contribute to metastatic spread outside of the brain2. As endothelial activation is a hallmark of neuroinflammation and vascular cell adhesion molecule-1 (VCAM-1) is a key mediator of such inflammation, our objective was to determine whether brain metastases formation is aided by VCAM-1 upregulation due to pre-existing inflammation.Methods
Imaging VCAM-1. Inflammation was induced in Balb/c mouse using stereotaxic lipopolysaccharide (lipopolysaccharide (LPS), 1 µg in 1 µl saline, n = 3) injection into the right hemisphere (RH) and evaluated 24 h later using the distribution of VCAM-1 as a marker of vascular inflammation. Mice injected with saline or sham-operated animals were used as controls. VCAM-1 distribution was visualized and quantified using molecular magnetic resonance imaging (MRI) with VCAM-1 antibody conjugated microparticles of iron oxide (MPIOs) as first described by McAteer et al3.
Imaging metastases. VCAM-1 targeted MPIOs can also be used to detect metastases with high sensitivity, because VCAM-1 is expressed on vessels associated with metastases and by tumor cells4. Hence, to study the impact of pre-existing inflammation on the distribution of tumor cells, mice injected with LPS (or controls, as above) were injected intracardially with 4T1 breast cancer cells (105 cells in 100 µl phosphate buffered saline) 24 h later. Metastasis imaging was performed on days 10 and 18 post cell injection.
Exploring the role of VCAM-1 on metastases formation. An extra group of mice received an intravenous injection of VCAM-1-MPIOs 24h after intracerebral LPS-injection, putatively blocking VCAM-1 binding sites, followed by 4T1 cell injection 4 hours later. In this group MRI was performed 3.5 h after the injection of VCAM-1-MPIO (to detect inflammation), as well as on day 18 post tumor cell injection (to detect metastases). All MR experiments were conducted on a small animal 7T scanner (Varian Inc., Palo Alto, CA) with a dedicated mouse head-coil (RAPID MR International, OH) using a T2*-weighted sequence (TR = 50 ms, TE = 25 ms, flip angle = 15°, data matrix = 256x256x96, field of view = 20 x 20 x 10 mm3, 2 averages). Brains were extracted following the final imaging session for histological analysis.
Results
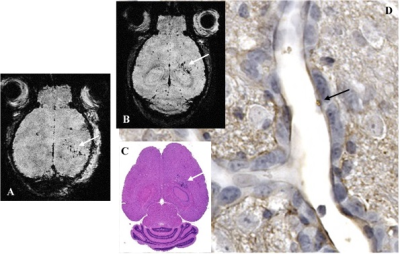
Figure 1A - Inflammation detection - Representative T2* weighted axial image of VCAM-1 in a mouse injected with VCAM-1-MPIOs 24h post LPS injection in the RH. Specific retention of the contrast agent on activated vasculature is visible as signal voids (white arrow) in the inflamed hemisphere but was hardly detected in the contralateral hemisphere. Figure 1B - Metastasis detection - Representative T2* weighted axial image of brain metastases on day 18 post cancer cells injection in an animal treated on day 0 with LPS. MR detection of brain metastases is confirmed by histological analysis (Fig. 1C). Metastases are detected through VCAM-1 expression in vessels associated with metastases as shown by VCAM-1 immunostaining (Fig. 1D, black arrow: VCAM-1-MPIOs bound to an activated vessel inside a tumor in Fig. 1C).
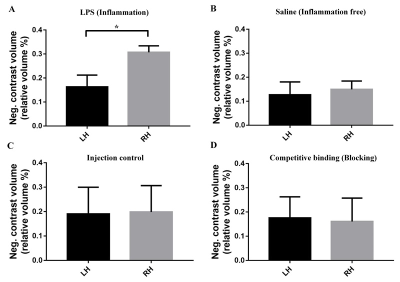
The number of brain metastases was markedly increased (2 fold higher) in the inflamed hemisphere (Fig. 2A) compared to the contralateral hemisphere and to control conditions (Fig 2B and C). The distribution of metastases co-localize with inflammation (Fig. 1A, B, C white arrow). Evidence that VCAM-1 contributes to tumor cell adhesion was demonstrated by functionally blocking VCAM-1 with anti-VCAM-1 MPIOs pre-treatment in LPS treated animals. This blocking reduces the number of metastases in the inflamed hemisphere back to control values (Fig. 2D). MRI results were confirmed by tumor quantification on histological sections (data not shown).
Discussion and Conclusion
This study validates the use of targeted MPIOs to non-invasively study (1) the role of pre-existing cerebrovascular inflammation via cell adhesion molecules, here VCAM-1, in the genesis of brain metastases and (2) the detection of brain metastases. Our findings suggest that pre-existing inflammatory conditions (e.g., radiation therapy, neurodegenerative diseases) might greatly increase the risk of brain metastases and highlight the need for preventive approaches targeting inflammation in vulnerable patients.Acknowledgements
No acknowledgement found.References
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 144:646–674 (2011).
- Ferjanćić, S., Gil-Bernabé, A.M., Hill, et al. VCAM-1 and VAP-1 recruit myeloid cells that promote pulmonary metastasis in mice. Blood 121, 3289–3297 (2013).
- McAteer MA, Sibson NR, von zur Muhlen C, et al. In vivo magnetic resonance imaging of acute brain inflammation using microparticles of iron oxide. Nature Medicine 13, 1253 - 1258 (2007).
- Serres S, Soto MS, Hamilton A, et al. Molecular MRI enables early and sensitive detection of brain metastases. Proc Natl Acad Sci U S A. 109(17):6674-6679 (2012).
Figures