0629
Collagen is a major determinant of the viscoelastic properties of stromal-dense tumours: insights from pre-clinical MRE1Division of Radiotherapy & Imaging, The Institute of Cancer Research, London, United Kingdom, 2Division of Molecular Pathology, The Institute of Cancer Research, London, United Kingdom, 3Division of Imaging Sciences and Biomedical Engineering, King's College London, King's Health Partners, St. Thomas' Hospital, London, United Kingdom
Synopsis
The relationship of magnetic resonance elastography-derived viscoelasticity quantified in vivo in nine pre-clinical tumour models exhibiting varying degrees of stromal density was compared with histological assessments of cellularity, collagen and vessel density. Both cellularity and collagen deposition were identified as important determinants of the relative tumour stiffness.
Introduction
The altered extracellular matrix (ECM) and stromal composition associated with the tumour microenvironment results in aberrant tensional homeostasis. The ensuing increase in tissue stiffness contributes to tumour progression1, hence approaches that modulate the ECM for therapeutic gain are being actively pursued2. Methods that accurately quantify tumour stiffness in vivo may thus provide prognostic biomarkers of tumour progression, and prove useful for monitoring treatment response.
Magnetic resonance elastography (MRE) is an emerging imaging technique being used to directly visualise and quantify the viscoelastic properties of tumours in vivo, both in pre-clinical tumour models3,4 and in cancer patients5,6.
The relationship between MRE measurements of viscoelasticity and tumour stromal components must be established and validated before they can be routinely deployed as imaging biomarkers of response to stromal targeting therapies. In this study, MRE-derived viscoelastic measurements acquired in vivo from nine pre-clinical tumour models exhibiting varying degrees of ECM/stromal density were compared with quantitative histological measurements of cellularity, collagen and vascular density.
Methods
All experiments were performed in accordance with the UK Animals (Scientific Procedures) Act 1986. Data were acquired from mice bearing orthotopic MDA-MB-231 (n=5) or BT474 (n=7) breast tumours, orthotopic PANC-1 pancreatic ductal adenocarcinomas (n=7), intracranial tumours derived from U87-MG (n=8), RG2 (n=7) or D212-MG (n=7) glioma cells or MDA-MB-231 breast tumour cells (n=5), neuroblastomas (n=9) spontaneously arising in Th-MYCN transgenic mice, and medulloblastomas (n=10) arising in GTML/Trp53KI/KI transgenic mice.
MRE data were acquired using a 3cm birdcage coil on a 7T Bruker MicroImaging horizontal MRI system (Bruker Instruments, Ettlingen, Germany). MRE data was acquired in the axial plane using a purpose built platform as previously described4. Maps of the absolute value of shear stiffness |G*| (kPa), were reconstructed with an isotropic pixel size of 300µm.
Paraffin-embedded tissue sections were subsequently cut and stained with H&E or picrosirius red for the assessment of cellularity and collagen types I & III respectively. Additional sections were immunohistochemically processed for detection of the endothelial cell marker endomucin for the assessment of microvessel density. H&E stained histopathological images were automatically segmented using CRImage7 and cellular components were identified. Quantitative analysis of picrosirius red staining and endomucin staining was performed using ImageJ, enabling determination of the percentage stained area of each sample.
Results
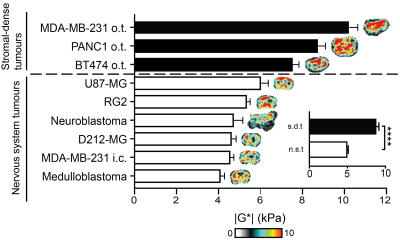
A wide range of values of |G*| was determined across the range of tumour models investigated, ranging from ~4 to ~10kPa (Figure 1). Interestingly, tumour stiffness was markedly elevated in stromal-dense orthotopic breast and pancreatic tumours, whereas the remaining tumours originating from the nervous system were relatively softer. Comparison of |G*| between these two groups revealed that the stromal-dense tumours were significantly stiffer (Figure 1. inset).
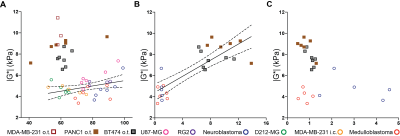
A significant yet weak relationship between |G*| and cellularity was identified for the nervous system tumours only, whereas a positive and statistically significant correlation was found between |G*| and collagen content across all the tumour types (Figure 2A&B). The highest microvessel density was found in hypervascular neuroblastomas.
Discussion
This study demonstrates the ability of MRE to quantify viscoelasticity across a diverse range of tumour types/histopathologies in vivo, and reinforces the role of collagen (type I & III) as a major determinant of the elevated stiffness of stromal-dense tumours. There is an extensive body of work that shows how collagen and its microscopic structural complexity is responsible for reduced tumour interstitial macromolecular transport and enhanced cancer cell dissemination8,9. We recently reported a rapid (5 hours) and marked reduction in |G*| (~2kPa) in BT474 breast tumour xenografts following administration of collagenase10, providing preliminary evidence for the sensitivity of MRE to collagen-mediated changes in viscoelastic properties. Further MRE/histology cross validation studies are needed to understand regional variations in |G*| in relation to collagen (and its structural complexity) in order to evaluate the ability of |G*| to map the intricacy of collagen deposition in tumours.
Conclusion
This study reinforces the ability of MRE to non-invasively detect change in tissue microstructure, more specifically changes in stromal microstructure and warrants further investigations to evaluate the potential of MRE-derived viscoelastic biomarkers as predictive/prognostic biomarker of response to stroma-targeted therapy in stromal-dense tumours.Acknowledgements
We acknowledge funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 668039, Rosetrees Trust grant A1091, Cancer Research UK support to the Cancer Imaging Centre at ICR in association with MRC and Department of Health C1060/A16464 and NHS funding to the NIHR Biomedicine Research Centre, and a Children with Cancer UK Research Fellowship.References
1. Paszek, M.J., et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241-254.
2. Cox, T.R. & Erler, J.T. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech. 2011;4:165-178.
3. Jamin, Y., et al. Exploring the biomechanical properties of brain malignancies and their pathologic determinants in vivo with magnetic resonance elastography. Cancer Res. 2015;75:1216-1224.
4. Li, J., et al. Tumour biomechanical response to the vascular disrupting agent ZD6126 in vivo assessed by magnetic resonance elastography. Br J Cancer. 2014;110:1727-1732.
5. Sinkus, R., et al. Viscoelastic shear properties of in vivo breast lesions measured by MR elastography. Magn Reson Imaging. 2005;23:159-165.
6. Simon, M., et al. Non-invasive characterization of intracranial tumors by magnetic resonance elastography. New Journal of Physics. 2013;15:085024.
7. Yuan, Y., et al. Quantitative image analysis of cellular heterogeneity in breast tumors complements genomic profiling. Sci Transl Med. 2012;4(157):143.
8. McKee, T.D., et al. Degradation of fibrillar collagen in a human melanoma xenograft improves the efficacy of an oncolytic herpes simplex virus vector. Cancer Res. 2006;66:2509-2513.
9. Egeblad, M., Rasch, M.G. & Weaver, V.M. Dynamic interplay between the collagen scaffold and tumor evolution. Curr Opin Cell Biol. 2010;22:697-706.
10. Li, J., et al. Imaging Collagenase-Induced Changes in the Mechanical Phenotype of Orthotopic BT474 Breast Cancer Xenografts Using Magnetic Resonance Elastography. Proc. ISMRM 25. 2017:4485.
Figures