0628
Radiated Brain Microenvironment Enhances Glioma Growth and Blunts Immunotherapy1Washington University in St. Louis, Saint Louis, MO, United States
Synopsis
Glioblastoma (GBM) is a highly aggressive, malignant, primary brain tumor. Despite state-of-the-art standard-of-care treatment (surgery, chemotherapy, radiation), GBM inevitably recurs, usually in the peritumoral irradiated tumor/brain interface within two centimeters of the margins of the resection cavity. Anti-PD-L1 (immune checkpoint) inhibitors represent an important new class of cancer treatments, but have shown little efficacy in GBM. In a mouse glioma model, we demonstrate that late evolving effects of radiation blunt the therapeutic effectiveness of anti-PD-L1. Carbogen/O2 gas challenge experiments in these mice six weeks post-irradiation demonstrate that the brain microenvironment is physiologically modified, consistent with the observed blunting of immunotherapy.
Purpose:
Glioblastoma (GBM) is a highly aggressive, malignant, primary brain tumor (1). Maximal neurosurgical tumor resection, followed by highly conformal radiation and concurrent chemotherapy, remains the standard of care for glioblastoma (GBM) patients (2). Despite state-of-the-art treatment, these tumors inevitably recur, the vast majority in the peritumoral irradiated tumor/brain interface within two centimeters of the margins of the resection cavity (3, 4).
Programmed cell-death protein 1 (PD-1) is a checkpoint protein on T cells that helps prevent these cells from attacking other cells by binding to a ligand, PD-L1. By blocking PD-1 on T cells or PD-L1 on cancer cells, checkpoint inhibitors enable T cells to attack the cancer. In GBM, the PD-1/PD-L1 immunotherapy pathway has not shown the promising clinical success seen in other cancers. Herein, we present striking evidence that the delayed effects of previous brain-tissue irradiation may explain the failure of many potential therapeutic agents to translate successfully from in vitro studies to the clinic.
Methods:
Mouse Irradiation. Naïve (non-irradiated) GL261 tumor cells were implanted into the ipsilateral hemisphere of brains of C57BL/6 mice that had been hemispherically irradiated (Gamma Knife) six weeks earlier (prior to implantation) with a single fraction, 30 Gy, 50% isodose, and into a group of non-irradiated mice as control. Mice show no frank appearance of radiation necrosis at this irradiation dose (i.e., the irradiated hemisphere appears normal). Immunotherapy. Beginning on post-implantation day 3, mice were treated IP every three days, for a total of five treatments, with an anti-PD-L1 mouse monoclonal antibody, or DMSO vehicle control. MRI. Images were acquired with a 4.7-T small-animal Agilent/Varian DirectDriveTM scanner using actively decoupled transmit and receive coils. Multislice, post-contrast T1-weighted, spin-echo transaxial images were acquired for mice in both experiments: TR = 650 ms; TE = 16 ms; FOV = 15 x 15 mm2; slice thickness = 0.5 mm; 21 slices to cover the whole brain. T2-weighted, spin-echo transaxial images were acquired with the same field of view: TR = 2000 ms; TE = 30 ms. In the breathing-gas challenge experiments, mice alternately breathed a mildly hypoxic (12.5% O2) gas mixture or carbogen (95% O2; 5% CO2).Results:
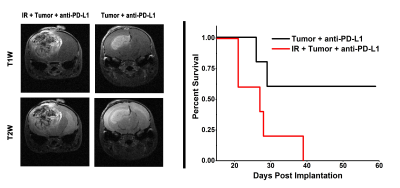
Representative MR images of anti-PD-L1-treated mice, collected on post-implantation day 21 (nine weeks post irradiation for the irradiated group) are shown in Fig. 1, left panel; corresponding Kaplan-Meier curves are shown in Fig. 1, right panel. The results are striking. Tumors originating from naïve (non-irradiated) tumor cells implanted in the absence of prior brain irradiation responded much more robustly to immunotherapy than naïve tumors implanted in previously (six weeks prior to implantation) irradiated brain. Tumors growing in a non-irradiated microenvironment are significantly smaller in anti-PD-L1-treated animals, and survival is extended appreciably relative to mice with tumors growing in previously irradiated (six weeks prior to implantation) microenvironment. In short, the irradiated microenvironment of the brain substantially blunts the therapeutic effectiveness of immunotherapy in mice.Discussion:
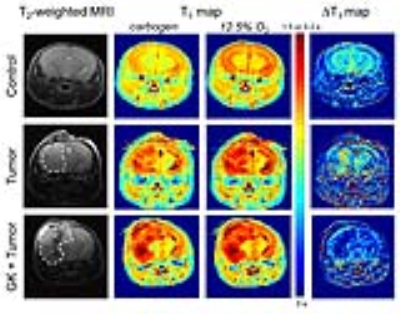
In patients, recurrent GBM, growing in previously irradiated brain tissue, is more aggressive, invasive, and hemorrhagic than primary tumor, and these recurrent tumors do not respond to checkpoint inhibition. These effects are accurately reproduced in our mouse model, in which naïve (non-irradiated) tumor cells are implanted into previously irradiated mouse brain. It is important to emphasize that in all of these experiments, naïve tumors cells were implanted six weeks post-irradiation, long after the acute effects of the radiation had dissipated, analogous to the regrowth of high-grade brain tumor in patients following chemo-radiation treatment (re standard of care). That the radiation modulated microenvironment six weeks post-irradiation is physiologically modified, despite the absence of frank signs of necrosis, is seen in its response to carbon/O2 challenge (Fig. 2).Conclusions:
In a mouse glioma model, late evolving effects of radiation blunt the therapeutic effectiveness of immunotherapy, findings that are potentially profound with respect to patient care. This mouse model is a valuable platform for a wide variety of studies aimed at understanding the virulence of recurrent tumor growth and its resistance to immunotherapy, including checkpoint inhibition. This enhanced understanding may lead to improvements in the dose/timing of radiation treatment, alone and in combination with other therapies that will translate to improved patient care and survival.Acknowledgements
This work was supported by NIH grant R01 CA155365 (JRG), funding from the Alvin J. Siteman Cancer Center (P30 CA091842), Mallinckrodt Institute of Radiology, the Barnes-Jewish Hospital Foundation Cancer Frontier Fund, and Elekta Instruments AB (Stockholm, Sweden).References
1. Barajas RF, Jr., Phillips JJ, Parvataneni R, Molinaro A, Essock-Burns E, Bourne G, et al. Regional variation in histopathologic features of tumor specimens from treatment-naive glioblastoma correlates with anatomic and physiologic MR Imaging. Neuro-oncology. 2012;14:942-54.
2. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England journal of medicine. 2005;352:987-96.
3. Chan JL, Lee SW, Fraass BA, Normolle DP, Greenberg HS, Junck LR, et al. Survival and failure patterns of high-grade gliomas after three-dimensional conformal radiotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20:1635-42.
4. Liang BC, Thornton AF, Jr., Sandler HM, Greenberg HS. Malignant astrocytomas: focal tumor recurrence after focal external beam radiation therapy. Journal of neurosurgery. 1991;75:559-63.
Figures