0627
Dynamic 23Na-Imaging of the Human Lung with Fully Flexible Intrinsic Respiratory Gating1Division of Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 2Institute of Radiology, University Hospital Erlangen, Erlangen, Germany, 3Diagnostic and Interventional Radiology, University Hospital of Heidelberg, Heidelberg, Germany
Synopsis
23Na lung MRI is a potential tool e.g. for investigating tumor viability and treatment response. The acquired data is, however, influenced by respiratory motion since breath-hold acquisitions are not possible due to the long measurement times. Here, we present a method for full flexibility in retrospective gating of 3D-radial data, combined with a Compressed-Sensing reconstruction. Up to 14 frames could be reconstructed from one in-vivo dataset, corresponding to a temporal resolution of 0.27 s. The temporal resolution of 23Na lung MRI could potentially lead to a more accurate quantification of the Tissue Sodium Concentration in the human torso.
Introduction
23Na lung MRI has proven to be a potential tool for investigating lung tumor viability and treatment response1 as well as fluid distribution in lung edema2,3,4. Low in-vivo concentrations and low NMR sensitivity, however, are still a limiting factor for 23Na lung imaging. This can partly be overcome by measuring at ultra-high field, with dedicated hardware and pulse sequences, as well as by use of a compressed-sensing-based image reconstruction. Respiratory motion represents another challenge for 23Na lung imaging, especially since breath-hold acquisitions are not realistic due to the long measurement times. Here we propose a fully flexible retrospective intrinsic data sorting in combination with a compressed sensing reconstruction of the resulting subsets in order to minimize motion-induced blurring5,6.Methods
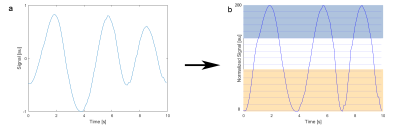
A healthy volunteer (male, 30y) was examined in a 7T whole-body MR scanner (MAGNETOM 7T, Siemens Healthcare, Erlangen, Germany) using a 4-port oval-shaped birdcage coil7. A 3D density-adapted radial UTE sequence8 with golden angle projection distribution9 was employed for data acquisition (TE / TR = 0.85ms / 20ms, nominal spatial resolution (Δx)3 = (4mm)3, NProjections = 18200, fivefold short-term averaging, TAQ = 30 min 20 s). The signal intensity at k-space center was used for the assessment of respiratory motion10. The zero crossings of the signal were used to identify breathing cycles. The intrinsically gated respiratory signal of each breathing cycle was then normalized and separated into 200 equidistant bins (Figure 1b). Out of these bins, a set of starting points for the reconstruction of the frames in the time domain was defined (Figure 1c). From these starting points, projections were added from following bins until a predefined minimal number of projections is reached, ensuring that each subset contains approximately the same number of projections. This processing leads to overlapping subsets of projections, starting from the fully inhaled state, gradually proceeding in a sliding window towards the fully exhaled state. The free selection of the frame starting points and of the number of minimal projections allows the reconstruction of any number of time frames with varying overlap. The resulting subsets are then reconstructed with the 3D-Dictionary-Learning-Compressed-Sensing algorithm (3D-DLCS)11 (block size B = 5, dictionary size D = 400, sampling number Nsamp = 500000, weighting of data-consistency λ = 0.5) to reduce resulting undersampling artifacts.Results
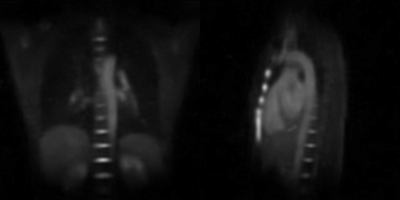
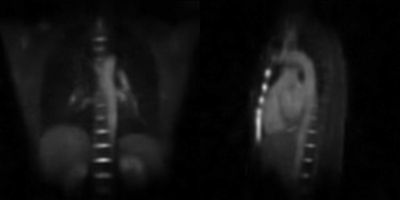
The reconstructions resulting from using 12 starting points and a minimal number of 7000 projections for each subset (undersampling factor USF ≈ 4.5) are shown in the animated gif from Figure 2. The respiratory states are well separated, and undersampling artifacts are minimal with negligible noise. 23Na signal variations are especially pronounced at the border of the liver, but also in coronary vessels and the heart itself. Another reconstruction using 14 starting points and a minimum of 5000 projections (USF ≈ 6.3) is shown in the animation in Figure 3. Here, the image quality starts to be affected by the increased temporal resolution, leading to residual artifacts. The mean duration of each respiratory cycle for the volunteer was Tresp = 3.77 s. For the 14-frame reconstruction, the frame temporal resolution therefore computes to Δt = 0.27 s, whereas Δt = 0.32 s for the 12-frame reconstruction.Discussion & Conclusion
We present a method for the reconstruction of intrinsically-gated 23Na lung MRI data with full flexibility in the temporal domain. The reordering of the data is obtained by selecting starting points within the 200-bin histogram of the respiratory signal obtained from the signal intensity at k-space center and setting a minimum number of projections to be reached in each data subset. The sensitivity achieved to respiratory motion in 23Na lung MRI can be essential for quantification of Na+ in tumorous tissue, pulmonary edema, and the myocardium12.Acknowledgements
This work was funded in part by the Helmholtz Alliance ICEMED - Imaging and Curing Environmental Metabolic Diseases, through the Initiative and Networking Fund of the Helmholtz Association. This study was also supported by grants from the German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung, BMBF) to the German Center for Lung Research (Deutsches Zentrum für Lungenforschung, 82DZL00401 and 82DZL004A1).References
1) Henzler, T. et al. Rofo (2012) 184: 340-4
2) Kauczor, H.U. & Kreitner, K.F. Eur Radiol (1999) 9: 1755-64
3) Kundel, H.L. et al. Magn Reson Med (1988) 6: 381-9
4) Lancaster, L. et al. Magn Reson Med (1991) 19: 96-104
5) Madelin, G. & Regatte, R.R. J Magn Reson Imaging (2013) 38: 511-29
6) Lustig, M. et al. Magn Reson Med (2007) 58: 1182-95
7) Platt, T. et al. Proc Intl Soc Mag Reson Med (2017) 25: 2958
8) Nagel, A.M. et al. Magn Reson Med (2009) 62: 1565-73
9) Chan, R.W. et al. Magn Reson Med (2009) 61: 354-63
10) Platt, T. et al. Proc Intl Soc Mag Reson Med (2017) 25: 0486
11) Behl, N.G.R. et al. Magn Reson Med (2016) 75 : 1605-16
12) Thulborn, K.R. NeuroImage (2016) https://doi.org/10.1016/j.neuroimage.2016.11.056
Figures