0625
Metabolic Rate of Oxygen Consumption in Brain Tumors: A Pilot 17O-MRI StudySebastian C. Niesporek1, Armin M. Nagel1,2, Reiner Umathum1, Nicolas G.R. Behl1, Mark E. Ladd1, Heinz-Peter Schlemmer3, and Daniel Paech3
1Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 2Institute of Radiology, University Hospital Erlangen, Erlangen, Germany, 3Division of Radiology, German Cancer Research Center (DKFZ), Heidelverg, Germany
Synopsis
The cerebral metabolic rate of oxygen (CMRO2) is an interesting biomarker and can be used as a diagnostic parameter in various neurodegenerative diseases or tumors. A dynamic 17O MRI inhalation method was optimized for application in a clinical setting and employed in two patient examinations to investigate CMRO2 in human brain tumors as part of an ongoing pilot study. In tumor tissue, a decrease in oxygen consumption was detected, which is in consistent with the Warburg effect. To our knowledge, the presented work includes the first patient study with dynamic 17O MRI.
PURPOSE
In the energy metabolism of most organisms molecular oxygen (O2) plays a vital role. In various diseases such as cancer (‘Warburg effect’) [1], Parkinson’s [2], or Alzheimer’s disease [3] the functional parameter of cerebral metabolic rate of oxygen consumption (CMRO2) contains information about tissue viability. The CMRO2 parameter can be measured via dynamic 17O MRI of the stable oxygen isotope 17O employing an inhalation procedure where enriched 17O2 gas is administered during continuous imaging [4]. Recently, the reproducibility and reliability of CMRO2 determination via 17O MRI with additional partial volume (PV) correction was demonstrated in a small volunteer cohort [5]. The presence of strong PV effects in 17O-MRI is caused by low spatial resolution and rapid transverse relaxation (T2~5ms), requiring an efficient correction procedure to separate signal contribution from different compartments. In the presented work the verified method from [5] was utilized in a clinical setting to measure two tumor patients and quantifying CMRO2 values in various regions in healthy tissue and tumor regions.METHODS
A three-phase inhalation experiment [4] (t1 baseline-phase; t2 17O-inhalation-phase; t3 decay-phase) was performed with a MR‑compatible breathing system in which a 17O2-bolus is administered in a closed circuit. In this ongoing IRB approved study one untreated glioma-patient (male, 26y.-o. astrocytoma WHO grade II) and one untreated glioblastoma-patient (male, 34y.-o., WHO grade IV) were included. 17O MRI was conducted at a 7 Tesla whole-body scanner (Siemens Healthineers, Erlangen, Germany) with a nominal spatial resolution of (7.5mm)3 employing a density-adapted radial sequence [6] with Golden Angle acquisition scheme [7] (TR/TE=20/0.56ms, total acquisition time 30:00min). Data were reconstructed with a temporal resolution of Δt=1:00min. Per experiment 3.8±0.1L of 70%-enriched 17O2 gas (NUKEM Isotopes Imaging GmbH, Alzenau, Germany) were administered. High-resolution T1 MPRAGE (0.6mm)3 and T2 TSE (0.4x0.4x0.5mm)3 sequences were additionally acquired and used for tumor and normal brain segmentation for the employed PV correction algorithm [8,9]. Prior to patient measurements the imaging protocol was optimized utilizing dynamic numerical signal simulation of the human brain: the impact of shortening of the baseline- and decay-phases on the accuracy of quantification was investigated and the final patient protocol was adjusted accordingly.RESULTS
The available fit information in individual breathing phases (t1, t3) was systematically reduced in two considered compartments (gray matter (GM), white matter (WM)). The deviation from the simulation ground truth is shown in Fig.1. Only a minor dependence is seen for variation of t1 and a deviation >5% for t3<15min. With this information the patient inhalation protocol was set to: t1=5:00min, t2≤10:00min (variable until 17O2 is exhausted) , t3≤15:00min. Results of the glioma WHO II -patient revealed significantly decreased CMRO2 in tumor tissue (0.59–0.66±0.16μmol/g/min) compared to contralateral normal appearing brain tissue (1.10–1.22±0.08 μmol/g/min, control compartment, Tab.1). Quantitative data are shown in Fig.2 and a map of relative 17O signal increase visualizes the decrease of oxygen metabolization in the tumor region (Fig.3). Further, CMRO2 was significantly increased in gray matter (2.34–2.58±0.20μmol/g/min) compared to white matter tissue (0.56–0.62±0.06μmol/g/min) after application of PVC. CMRO2 determination in the glioblastoma patient, revealed an even more pronounced metabolization drop in the tumor tissue, with lowest values in the necrotic (NE) as well as contrast enhancing (CE) tumor region (Tab.1).DISCUSSION
Application of a simulation tool allowed protocol optimization and estimation of expected fitting errors due to information reduction in individual breathing phases. The protocol and utilized inhalation setup allowed measurements in two patients and show the feasibility of transferring the suggested method to a clinical environment. The drop in quantified functional parameters in the malignant tissue (CE, NE, PE) corresponds to the Warburg effect, which describes decreased CMRO2 in tumors due to the shift in glucose metabolism from oxidative phosphorylation to lactate production for energy generation as reported for 17O MRI data for the first time by Hofmann et al. [10]. Overall functional parameters are in good agreement with previous reports (Tab.1) [10, 11]. Due to large voxel volumes and rapid transverse relaxation, the quantified H217O concentrations might be underestimated despite the application of a dedicated PVC algorithm. Uncertainties in the prior 17O enrichment factor as well as limitations of the PVC as discussed in [5] are the main sources of error in the suggested method. However, CMRO2 values of healthy tissue (GM, WM) and cerebrospinal fluid (CSF) are in correspondence with previous work [4,5].CONCLUSION
This work presents the first results of an ongoing study investigating brain tumor metabolism in glioma-patients with the help of dynamic 17O MRI. Further application may provide new insights into tumor pathophysiology through the visualization of the cerebral metabolic rate of oxygen consumption.Acknowledgements
The authors want to thank NUKEM Isotopes Imaging GmbH for their generous supply of 17O2-gas and support of this project.References
[1] Miles KA, Williams RE., Cancer Imaging 2008 (8):81-86; [2] Beal MF., Ann Neurol; 1992 (2):119-130; [3] Maurer I, Neurobiology of Aging 2000;21(3):455-462; [4] Atkinson IC, Neuroimage 2010 (51):723-733; [5] Niesporek SC, Magn Reson Med 2017, doi: 10.1002/mrm.26952; [6] Nagel et al., Magn Reson Med 2009 (62):1565-73; [7] Chan, R.W. et al., Magn Reson Med 2009(61): p. 354–363; [8] Rousset et al., J Nucl Med 1998(5):904-911, 1998, [9] Niesporek et al., NeuroImage, 2015(112): 353–363; [10] Hoffmann et al., MAGMA 2014(27):579-87; [11] Ito, M., Neuroradiology 1982 (23): 63–74Figures
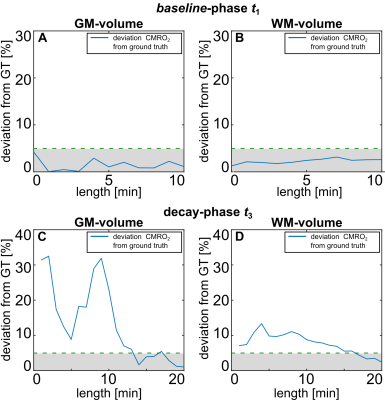
Fig. 1 Results of simulation study: variation of fit information in the
baseline-phase and decay phase (t1, t3) led to variation of deviation from
simulation’s ground truth (GT). Reduction of information led to variation <5% if
t3 is shortened from t3=20:00min to t3≥15:00min. Results led to adaption of the
patient protocol as mentioned in the main text.

Fig. 2 Quantitative signal evaluation during an inhalation experiment (glioma
WHO II patient) after employment of
the PV correction algorithm for gray matter (GM), white matter (WM) and tumor
volume (TV). The functional values can be found in Tab.1.
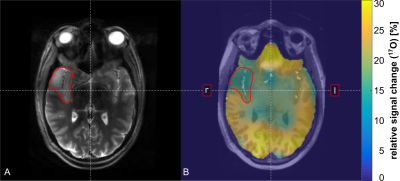
Fig. 3 Patient data: anatomical TSE data (A) and visualization of relative 17O signal increase (B) in a selected transversal slice. In (B), 17O data are shown as an overlay on anatomical
MPRAGE. The tumor volume (TV) was segmented manually and is indicated in both
images.
Tab. 1 Results of CMRO2 determination
of the patient study and comparison to previous work: the tumor volumes in both
patients were segmented differently. The malignant tissue for the glioma patient
was considered as one volume (tumor volume, TV). Additionally, as a control
volume a contralateral normal appearing brain tissue volume was evaluated for both patients. Segmentation for
the glioblastoma-patient included separation of the tumor volume into two
separately considered sub-volumes (necrotic region (NE), contrast-enhancing
region (CE)) as well as a third edema compartment (PE). In a separate
evaluation CE- and NE-volumes were considered as a TV-compartment of the
glioblastoma patient.