0624
Dynamic perfusion and T2*-weighted 1H MRI interleaved with multivoxel 31P MRS of exercising human calf at 7T1Center for Medical Physics and Biomedical Engineering, Medical University of Vienna, Vienna, Austria, 2MR Center of Excellence, Medical University of Vienna, Vienna, Austria, 3Centre for Clinical Magnetic Resonance Research, University of Oxford, Oxford, United Kingdom, 4Department of Biomedical Imaging and Image-guided Therapy, Medical University of Vienna, Vienna, Austria, 5NMR laboratory, Institute of Myology, Paris, France
Synopsis
To quantify perfusion and high-energy metabolite time courses of exercising gastrocnemius and soleus muscles in one time-resolved acquisition, 1H arterial spin labeling with multislice EPI was interleaved with multivoxel semi-LASER 31P-MRS. Perfusion mapping and T2* weighted MRI yielded reasonable time courses and were less sensitive to motion, compared to single slice acquisitions. Spectroscopic time courses of phosphocreatine, inorganic phosphate and pH with fitted PCr recovery time constants were consistent with literature in both GM and SOL. A significant 31P SNR increase was found when the VOI perceived adiabatic 1H inversion which can be explained by NOE.
Introduction
MR is a valuable tool to investigate dynamic changes of metabolically relevant parameters, in particular rates of oxidative phosphorylation in muscle tissue during exercise and recovery using 31P MRS1. On the other hand, arterial spin labeling in 1H-MRI is a non-invasive technique to quantify perfusion during and after stimulation without contrast agents2. It is possible to combine these two methods into a single time-resolved measurement, which has the advantage of giving a much more comprehensive picture on muscle metabolism by confronting information on high-energy metabolites with blood perfusion on oxygenation state3. Here, we present first results with an implementation of such a technique at 7T, using a technical modification to allow switching between different receiver frequencies4.
Methods
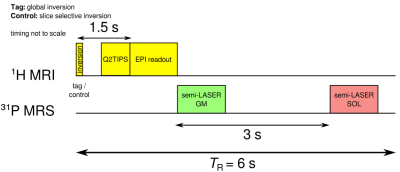
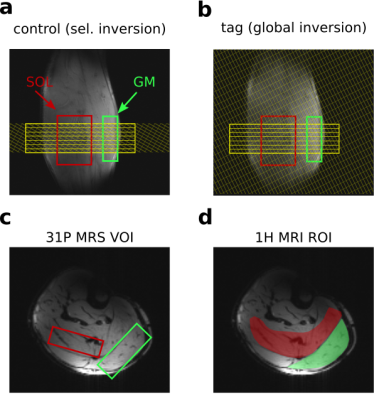
A pulsed arterial spin labeling (ASL) sequence with FAIR tagging scheme, SQ2TIPS and EPI readout5 was temporally interleaved with a multivoxel 31P semi-LASER acquisition scheme6 as shown in Figure 1. Seven EPI slices were acquired in sequential order (direction: H-F) without slice gaps (d=6mm each, TE=20ms). The slice group fully overlapped with the ASL selective inversion slab during control as shown in Figure 2a. TR was 6s, resulting in a set of perfusion images every 12s. Following the image readout, 31P spectra of gastrocnemius medialis (GM: 81cm³) and soleus (SOL: 67cm³) were acquired with a delay of 3 seconds (TE=29ms)6 see Figure 1. VOIs were placed as shown in Figure 2. Measurements were performed at 7T (Siemens, Erlangen, Germany) using a calf shaped multichannel 1H/31P transceiver surface coil-array7. The protocol comprised 2 minutes rest, 3 minutes plantar flexion exercise on a non-magnetic pneumatic ergometer, and 10 minutes recovery. A potential Nuclear Overhauser Effect (NOE) related 31P signal enhancement caused by 1H selective/global inversion was investigated in resting muscle. Images were reconstructed using Siemens’ ICE and evaluated using Matlab to calculate perfusion and T2* weighted time courses of both muscles using regions of interest as shown in Figure 2d. Processing of spectra was performed on raw data files using python for phasing and channel combination. Spectral quantification was done using jMRUI with AMARES followed by PCr recovery time fitting using gnuplot.Results
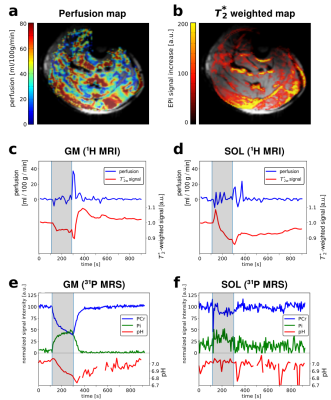
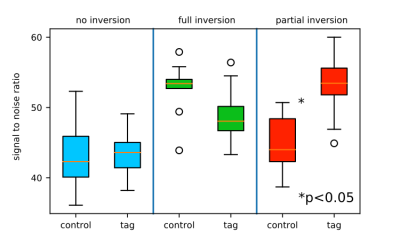
Perfusion, T2*-weighted MRI and phosphocreatine, inorganic phosphates and pH time courses were successfully quantified from a single exercise-recovery experiment. The perfusion map and the T2* weighted EPI images of a single healthy volunteer showing the difference between post exercise peak and rest are shown in Figure 3a,b together with the perfusion and T2* weighted time courses (mean over slice 2-4) of GM and SOL in Figure 3c,d. Mean perfusion was relatively stable during exercise and increased up to 37 ml/100g/min in GM and 24 ml/100g/min in SOL during recovery. The T2* weighted signal increased by 10% in GM and decreased by 12% in SOL during recovery compared to rest. PCr and Pi time courses (unaveraged) together with pH time courses (2x averaged) of GM and SOL are shown in Figure 3e,f. In GM, PCr decreased to 41% during exercise and end exercise pH reached 6.8 while SOL depleted to 87% only and pH stayed neutral. PCr recovery time constant τ was 33s in GM (with insufficient depletion in SOL for quantification of τ). The inversion of the proton spins had a significant impact on the 31P signal. SNR was significantly higher (p<0.05) if the 31P VOI perceived 1H inversion (Figure 4). In experiments where the selective inversion slab (control) did not affect the 31P VOI (i.e., by moving the inversion slab out of the VOI for test purposes), SNR was significantly lower compared to spectra acquired after global inversion.Discussion
Interleaving ASL and multivoxel 31P-MRS at 7T was successfully shown. The gap-less multislice EPI readout for ASL helped decrease motion related artifacts5 such as partial volume effects from neighbouring unsaturated spins moving into the imaging slice and resulted in high stability even during exercise. No signal loss was observed when combining the methods. Interestingly, the adiabatic inversion of the 1H-ASL part was found to increase the SNR in 31P-MRS, presumably due to NOE. In future experiments, dedicated NOE-pulses may be explored to further increase 31P- SNR independently of voxel and slice placement.Conclusion
This work demonstrates the successful implementation of time-resolved measurements of perfusion (1H-MRI) and of energy metabolism (31P-MRS) of different exercising human calf muscle groups simultaneously at 7T. This will improve the investigation of metabolic parameters in exercising human muscle tissue at ultra-high field by providing complementary information, enabling clinical applications.Acknowledgements
This work has been funden by the Austrian Science Fund (FWF): I1743-B13References
1. Fiedler GB, Schmid AI, Goluch S et al. Skeletal muscle ATP synthesis and cellular H+ handling measured by localized 31 P-MRS during exercise and recovery. Sci Rep 2016;6:32037
2. Frank LR, Wong EC, Haseler LJ et al. Dynamic imaging of perfusion in human skeletal muscle during exercise with arterial spin labeling. Magn Reson Med 1999;42:258-267
3. Carlier PG, Brillault-Salvat C, Giacomini E et al. How to Investigate Oxygen Supply, Uptake, and Utilization Simultaneously by Interleaved NMR Imaging and Spectroscopy of the Skeletal Muscle Magn Reson Med 2005;54(4):1010-1013
4. Meyerspeer M, Magill AW, Kuehne A et al. Simultaneous and Interleaved Acquisition of NMR Signals from Different Nuclei with a Clinical MRI Scanner Magn Reson Med 2016;76(5):1636-1641
5. Schewzow K, Fiedler GB, Meyerspeer M et al. Dynamic ASL and T2*- Weighted MRI in Exercising Calf Muscle at 7 T- A Feasibility Study Magn Reson Med 2015;73:1190-1195
6. Niess F, Fiedler GB, Schmid AI et al. Interleaved Multivoxel 31P MR Spectroscopy Magn Reson Med 2016;77:921-927
7. Goluch S, Kuehne A, Meyerspeer M et al. A Form-Fitted Three Channel 31P, Two Channel 1H Transceiver Coil Array for Calf Muscle Studies at 7 T Magn Reson Med 2015;73(6):2379-89
Figures