0623
Large FOV phosphor MR Spectroscopic imaging with multi-transmit proton MR imaging in the liver at 7 Tesla1University Medical Center Utrecht, Utrecht, Netherlands, 2University of Oxford Centre for Clinical Magnetic Resonance Research (OCMR), Oxford, United Kingdom
Synopsis
We combined 1H MRI and 31P MRSI in the liver by integrating a 31P RF body coil in a 7T MR system with a 16 channel 31P receive array merged with 1H fractionated dipole antennas The setup facilitates uniform 31P excitation by the body coil with high sensitivity from the receiver array, while providing B1 shimmed proton imaging with a dipole array.
Introduction
Conventional treatment of liver metastasis often results in palliative care as the effectiveness of the therapy can only be monitored after several months. Metabolite imaging with 31P MRSI is therefore an upcoming field in MRI as it can provide insight in tumor stage and therapy effectiveness, allowing for improved personalized treatment. However, current applications in 31P MRSI are limited by the use of surface transceivers consequently causing small field of views. In addition, the very high RF field inhomogeneity results in an uneven flip angle distribution. Adiabatic pulses to improve FA distribution are SAR demanding subsequently lowering the SNR. To overcome these limitations, body coils for uniform 31P transmit at 7T have been proposed1,2. In this work, we demonstrate the use of the 31P body coil with a 16 channel receiver array merged to eight fractional dipole antennas to combine metabolic imaging with conventional MRI.Methods
Phosphorus MRSI was performed using an in-house
designed detunable birdcage body coil integrated into
a 7T MRI system (Philips Healthcare, Best, Netherlands). A 16-channel body
array receiver tuned to phosphor at 7T was used for acquisition. To allow for simultaneous
proton imaging, eight fractionated dipole antennas were merged with the
phosphor receive array and driven by the multi-transmit system (Philips
Healthcare, Best, Netherlands)3. A male volunteer was positioned in the MR bore
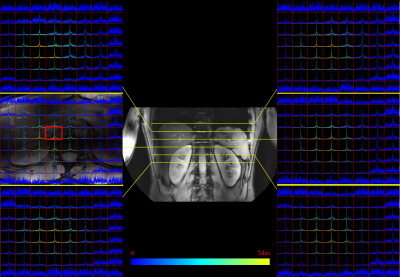
with the receive array wrapped around his torso and slightly skewed to the
right-side of the patient to properly cover the liver (Figure 1). Image based
B0 and B1 shimming was performed prior to calibration of the phosphor B1+
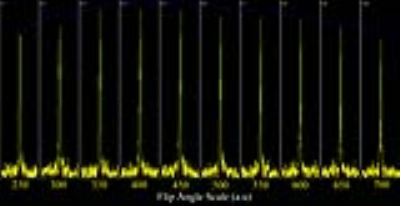
field, which was done by a flip angle sweep with scaling factors ranging from
250 to 750. Phosphor spectra were acquired using a 3D CSI sequence with an
isotropic resolution 24mm, a matrix size of 8x8x6 and 512 samples. Other
imaging parameters were BW, 8000Hz; TR, 2000ms; TE, 0.5409; NSA, 20; scan time,
20 minutes. Proton images were acquired using a multi-slice FFE with a total duration
of 23 seconds. Other imaging parameters were TR, 10ms; TE, 4.93ms; resolution,
0.78 x 0.78 x 10mm3; field-of-view, 500 x 500 x 30mm3 and
a flip angle of 15 degree. All data was processed in Matlab 2016b and data of
the receivers was combined using WSVD4.Results
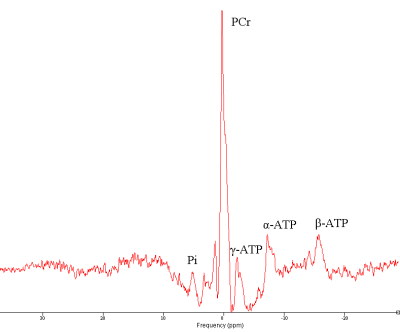
The flip angle series enables calibration of the RF power using 17KW as peak power (Figure 2). Coil combination was possible with the WSVD method. The integrated body coil in combination with the receive array for 31P MRSI showed high SNR (Figure 3) throughout the body. B0 shimming and partial volume effects were suboptimal over such large FOV as shown by the broad lines in the spectra and leakage of phosphocreatine (PCr) through all voxels (Figure 4). As the antennas are inherently decoupled from the 31P coils, good 1H MRI could be obtained (Figure 3, center and background).Discussion
Patient comfort during scanning is maintained by merging the 31P receiver array with the 1H transceiver array. Moreover, optimal 1H and 31P MRI can be obtained in one scan session without replacing RF coils. While we have shown a uniform transmit, high sensitive receiving merged with a 1H imaging setup, care must be taken in optimizing scan protocols for motion corrections and synchronized B0 shimming.Conclusion
Localized phosphorus MRSI by simultaneous proton imaging was successfully performed within a large field of view at 7 Tesla. Future clinical studies will benefit from the additional metabolite information gathered during imaging.Acknowledgements
No acknowledgement found.References
1. W. J. M. van der Kemp, V. O. Boer, P. R. Luijten, B. L. Stehouwer, W. B. Veldhuis, and D. W. J. Klomp, ‘Adiabatic multi-echo 31P spectroscopic imaging (AMESING) at 7 T for the measurement of transverse relaxation times and regaining of sensitivity in tissues with short T2* values’, NMR Biomed., vol. 26, no. 10, pp. 1299–1307, Oct. 2013.
2. L. Valkovič et al., ‘Using a whole-body 31P birdcage transmit coil and 16-element receive array for human cardiac metabolic imaging at 7T’, PloS One, vol. 12, no. 10, p. e0187153, 2017.
3. A. J. E. Raaijmakers et al., ‘The fractionated dipole antenna: A new antenna for body imaging at 7 Tesla’, Magn. Reson. Med., vol. 75, no. 3, pp. 1366–1374, Mar. 2016.
4. C. T. Rodgers and M. D. Robson, ‘Receive array magnetic resonance spectroscopy: Whitened singular value decomposition (WSVD) gives optimal Bayesian solution’, Magn. Reson. Med., vol. 63, no. 4, pp. 881–891, Apr. 2010.
Figures