0622
Creatine Kinase Rate Constant in the Human Heart at 7T: A Novel Superfast Magnetization Saturation Transfer Method1Department of Electrical and Computer Engineering, Auburn University, Auburn, AL, United States, 2Department of Biomedical Engineering, University of Alabama Birmingham, Birmingham, AL, United States, 3Siemens Healthineers and AUMRI Center, Auburn, AL, United States, 4Auburn University, Auburn, AL, United States
Synopsis
Changes in Creatine Kinase (CK) system are observed in heart failure and measurement of CK reaction rate constant (kf) can be useful to monitor pathology. 7T MR scanner offers improved signal to noise and spectral resolution and has potential for robust measurements. In this study we demonstrate novel superfast saturation transfer method (T1nom – method) to measure ATP production rate via CK forward reaction in human hearts at 7T. Spatial localization was achieved by GOIA-1D-ISIS/2D-CSI approach. Our measured kf values were consistent with literature.
Introduction
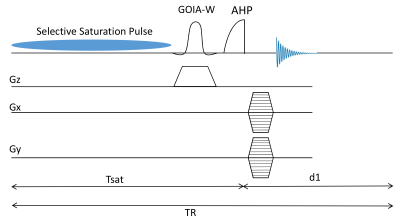
The creatine kinase (CK) enzyme catalyzes the reaction that converts adenosine diphosphate (ADP) and phosphocreatine (PCr) to adenosine triphosphate (ATP) and creatine (Cr). This facilitates the production and utilization of ATP by the contractile apparatus. The CK reaction rate can be measured noninvasively by the saturation transfer (ST) MRS techniques with and without spatial localization [1, 2]. Using the improved signal to noise offered by 7T magnet offers, 3D-CSI localized measurement of CK reaction rate constant in the myocardium were recently demonstrated [3]. However 3D-CSI localization results in long acquisition times and the technique also requires accurate calibration of flip angles using Bloch-Siegert shift which are not ideal for routine clinical research studies. We have previously demonstrated novel superfast saturation transfer method (T1nom – method) to measure transmural ATP production rate via CK forward reaction in pig hearts [4]. The aim of this study was to implement this approach at 7T for in vivo human heart. To avoid contamination of signal from the chest muscle we also incorporated GOIA-1D-ISIS/2D-CSI signal localization with the saturation transfer pulse sequence (Fig 1) [5].
Methods
T1nom method uses two partially relaxed spectra without (Mc) and with (Ms) saturation of ϒ-ATP saturation and the CK reaction rate (kf) constant is determined by
$$\frac{Mc}{Ms} = kf \times T1nom+1$$
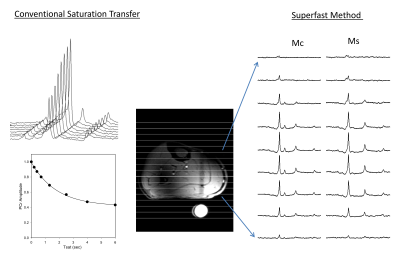
Spin parameters PCr:ATP = 0.5, T1 PCr = 4.5s, and T1 ATP = 1.8 s, kf CK = 0.3 1/s and kf ATP = 0.18 1/s were numerically simulated with Tsat = 1.8 s and d1 = 1 sec to determine T1nom using Bloch-Mconnell equations. All studies were performed on a Siemens 7T system (Erlangen, Germany) using a 31P/1H surface. Slice localization (3 cm) was achieved by 6 kHz bandwidth and 4ms duration GOIA-W (16, 4) inversion pulse in a 1D-ISIS scheme followed by 1D or 2D phase encoding gradients to obtain localized spectra. 1D-CSI was acquired with 16 phase encoding steps, FOV = 16 cm, spectral width = 6 kHz, averages = 16 and TE = 0.6 sec with 6 minute acquisition time. 2D-CSI was acquired with FOV = 16 cm x 16 cm, matrix = 16 x 8 and 16 averages with 13 minute acquisition time. ϒ-ATP saturation was achieved by repeated application of low powered continuous pulse (99 ms ON and 1 ms OFF) for total duration Tsat = 1.8 s. Spectra was analyzed in jMRUI using the advanced method for accurate, robust, and efficient spectral fitting (AMARES) [6] algorithm and kf was calculated using the above equation. Studies in calf muscle (n = 2) were used to test whether T1nom approach yield the same values for Kf as conventional saturation transfer method with TR = 12 sec and Tsat = 0.2, 0.4, 0.7, 1.3, 2.5, 4, 6 s. The technique was tested for human heart (n = 3).
Results
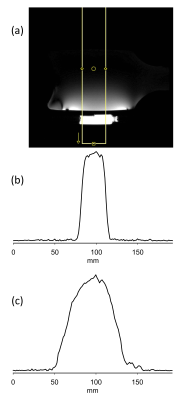
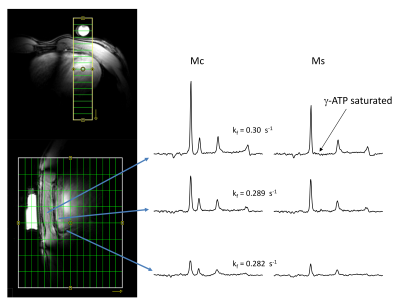
ISIS slice localization was determined in cylindrical sodium phosphate phantom and experimentally measured excitation profile of the slice is shown in Figure 2 showing excellent slice excitation with 31P surface coil and GOIA-W pulses. Non-localized plot (Fig 2c) shows signal drop off towards edges due to coil sensitivity profile. Skeletal muscle mean kf determined by conventional approach and T1nom approach were the same 0.278 ± 0.041 1/s and 0.282 ± 0.027 1/s demonstrating the validity of the technique (Fig 3). Figure 4 shows representative spectra from T1nom approach in the heart under partially relaxed conditions. The PCr signal is reduced when ϒ-ATP is saturated. The CK reaction rate constant shown in the figure agree with the previously reported values at 3T and 7T [6, 7]. When control irradiation is applied, no measurable decrease is seen in the PCr peak intensity is seen confirming that spillover is negligible at 7T due to increased spectral shift.Conclusions
In summary, we have demonstrated a novel steady-state saturation transfer method (T1nom) that lets accurate quantification kf under partially relaxed acquisition conditions. The new method allows fast kf measurement yet simple linear algorithm for quantification. We have combined this approach with GOIA-1D-ISIS/2D-CSI signal localization to enable fast localized kf measurement in heart at 7T. This method enables broad applications for in vivo enzyme kinetic studies that require high spatial or temporal resolution.Acknowledgements
We would like to thank Dr. Nelms and Auburn MRI center for support to complete this study.References
1. Bessman, S.P. and P.J. Geiger, Transport of energy in muscle: the phosphorylcreatine shuttle. Science, 1981. 211(4481): p. 448-52.
2. Bittl, J.A. and J.S. Ingwall, Reaction rates of creatine kinase and ATP synthesis in the isolated rat heart. A 31P NMR magnetization transfer study. J Biol Chem, 1985. 260(6): p. 3512-7.
3. Clarke, W.T., et al., Creatine kinase rate constant in the human heart measured with 3D-localization at 7 tesla. Magn Reson Med, 2017. 78(1): p. 20-32.
4. Xiong, Q., et al., ATP production rate via creatine kinase or ATP synthase in vivo: a novel superfast magnetization saturation transfer method. Circ Res, 2011. 108(6): p. 653-63.
5. Chmelik, M., et al., Fully adiabatic 31P 2D-CSI with reduced chemical shift displacement error at 7 T--GOIA-1D-ISIS/2D-CSI. Magn Reson Med, 2013. 69(5): p. 1233-44.
6. Vanhamme, L., A. van den Boogaart, and S. Van Huffel, Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson, 1997. 129(1): p. 35-43.
7. Bashir, A. and R. Gropler, Reproducibility of creatine kinase reaction kinetics in human heart: a (31) P time-dependent saturation transfer spectroscopy study. NMR Biomed, 2014. 27(6): p. 663-71.
Figures