0618
Towards Full-Brain FID-MRSI At 7T With 3D Concentric Circle Readout Trajectories1High Field MR Centre, Department of Biomedical Imaging and Image-guided Therapy, Medical University of Vienna, Vienna, Austria, 2Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Athinoula A. Martinos Center for Biomedical Imaging, Boston, MA, United States, 3Christian Doppler Laboratory for Clinical Molecular MR Imaging, Vienna, Austria, 4The Johns Hopkins University School of Medicine, Russell H. Morgan Department of Radiology and Radiological Science, Baltimore, MD, United States, 5Kennedy Krieger Institute, F. M. Kirby Research Center for Functional Brain Imaging, Baltimore, MD, United States
Synopsis
Proton magnetic resonance spectroscopic imaging is a powerful technique for clinical diagnosis; however, low spatial resolutions together with long scan times and SNR-per-time inefficiency prevent its wide spread application. The already proposed 2D-CONCEPT (concentric circle echo planar trajectories) sequence overcomes this issue by capitalizing most efficiently on the hardware constraints of the gradient system. The purpose of this abstract is to present first preliminary results of an extension to 3D-CONCEPT for full-brain 3D-FID-MRSI at 7T for a 64x64x31 matrix acquired within 23 minutes.
Introduction
Proton magnetic resonance spectroscopic imaging is a powerful technique for clinical diagnosis; however, low spatial resolutions together with long scan times and SNR-per-time inefficiency prevent its wide spread application (1). At favorable field strengths of 7T acceleration methods, which make use of spatial-spectral encoding suffer from increased spectral bandwidth demands, which pose challenges for available gradient hardware, but they enable far higher acceleration factors than parallel imaging (2,3). The already proposed 2D-CONCEPT (concentric circle echo planar trajectories) (4,5,6,7) sequence overcomes this issue by capitalizing most efficiently on the hardware constraints of the gradient system. As shown, 2D-CONCEPT not only outperforms other methods at 7T due to its self-rewinding nature, it provides also high SNR-per-time efficiency since its k-space density intrinsically resembles a low-pass filter. The purpose of this abstract is to present first preliminary results of an extension to 3D-CONCEPT for full-brain 3D-FID-MRSI at 7T for a 64$$$\times$$$64$$$\times$$$31 matrix acquired within 23 minutes.Sequences and Methods
All scans were
performed at a whole-body Siemens 7T MR scanner
with a 32-channel receive coil combined with a single Tx/Rx-element.
Due to the enormous raw data size acquired during each scan with 32
separate channels (~100-200GB), our first preliminary data here are
shown only using the single Tx/Rx channel. The local institutional
review board approved this study, and written consent was obtained.
The sequence was tested on phantoms and in vivo. The used 3D-MRSI sequence is based on a single-slice FID sequence (8) with a three-lobe sinc excitation pulse and a partition encoding gradient in slice direction (Figure 1, a). In-plane sine and cosine modulated gradients ensure the concentric circle readout. WET water suppression was used prior signal acquisition. A short pre-scan for noise-decorrelation was performed. MUSICAL coil combination (9) weights were obtained prior water-suppression by measuring the same trajectories as in the actual MRSI scan but with less FID points/circle circumnavigations and a flip angle of 5$$$^\circ$$$. In addition to 3D-MRSI, a MP2RAGE sequence was acquired for anatomical reference. $$$B_1^+$$$-mapping ensured the desired flip angle of 45$$$^\circ$$$. The scan parameters for 3D-CONCEPT were: TR 600$$$\,$$$ms, acquisition delay 1.3$$$\,$$$ms, matrix size 64$$$\times$$$64$$$\times$$$31, FOV 220$$$\times$$$220$$$\times$$$120 mm$$$^3$$$, VOI 220$$$\times$$$220$$$\times$$$50, $$$\,$$$mm$$$^3$$$ (Figure 1, b). The number of circles for the 31 partitions were: 1,12,16,20,22,24,26,28,29,30,31,31,32,32,32,32,32,32,32,31,31,30,29,28,26,24,22,20,16,12,1 (from north to south pole, Figure 2) in order to cover an ellipsoidal k-space volume for efficient filtering. The total measurement time was 23$$$\,$$$min 5$$$\,$$$s. 882 FID points were measured within three temporal interleaves resulting in a spectral bandwidth of 2778$$$\,$$$Hz.Reconstruction
Partition-wise 2D-gridding (10) using a Kaiser-Bessel kernel (kernel width of 3) was performed with an overgridding factor of 2. The reconstruction of the 3D-CONCEPT data used a modified Pipe-Menon (11,7) density compensation, which directly density compensates to Hamming weighted k-space partitions by choosing the appropriate weights. Since each k-space partition was sampled with a different number of circles (thus covering a different k-space extent / matrix size), zero-padding to a 64$$$\times$$$64$$$\times$$$1 matrix was necessary after gridding for 24 partitions (see above). Then stacking of all in-plane Fourier transformed partitions, together with cropping due to oversampling was performed. A final Hamming filtering and FFT in partition encoding direction provided a reconstructed 3D data set. All spectra were processed using simulated (8) LCModel basis sets including 17 metabolites and a measured macromolecule background (12).Results
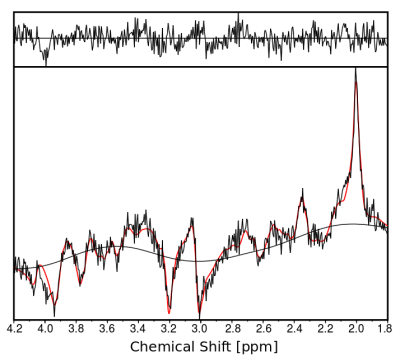
Figure 3 shows metabolic ratio maps of tCr/tNAA, Glx/tNAA, tCho/tNAA and Ins/tNAA for all three orientations and various slices (tCr=total creatine, tNAA=total N-acetyl-aspartate, Glx=glutamine + glutamate, tCho=total choline, Ins=Inositol). A sample spectrum of central gray matter is displayed in Figure 4.Discussion
3D-CONCEPT provides 3D metabolic ratio maps in all three orientations with excellent anatomical contrast. Currently, some maps contain voxels where the SNR was too low for reliable quantification due to usage of a volume coil only. A 3-5 times higher SNR can be expected as soon as all 32-Rx channels can be fully exploited as observed previously (9). Optimization of the memory-efficiency of our reconstruction algorithm will allow the processing of multi-channel coil data in the future. For an even better point-spread-function - and less lipid contamination - we will target sampling of a more spherical k-space via 63 partition encodings. Further work will also be necessary for the pulse profile homogeneity, which was currently tailored towards single-slice acquisitions with slabs ≤2cm. Even without parallel imaging we were 40$$$\times$$$ faster than traditional elliptical phase encoding.Conclusion
We have developed an SSE approach for 7T MRSI that combines SNR-efficiency and acquisition time-efficiency via optimal exploitation of the gradient hardware. Thereby, we achieved full-brain high-resolution 3D metabolic mapping in clinically attractive scan times of 23 minutes.Acknowledgements
This study was supported by the Austrian Science Fund (FWF): KLI-61 and the FFG project FA771E0801.References
(1) Oz G, Alger JR, Barker PB, et al. Clinical proton MR spectroscopy in central nervous system disorders. Radiology 2014;270:658–79.
(2) Strasser B, Považan M, Hangel G, Hingerl L, Chmelik M, Gruber S, Trattnig S, Bogner W. (2 + 1)D-CAIPIRINHA accelerated MR spectroscopic imaging of the brain at 7T. Magn Reson Med 2016.
(3) Hangel G, Strasser B, Považan M, Gruber S, Chmelík M, Gajdošík M, Trattnig S, Bogner W. Lipid suppression via double inversion recovery with symmetric frequency sweep for robust 2D-GRAPPA-accelerated MRSI of the brain at 7T. NMR Biomed 2015;28:1413–1425.
(4) Furuyama JK, Wilson NE, Thomas MA. Spectroscopic imaging using concentrically circular echo-planar trajectories in vivo. Magn Reson Med 2012;67:1515–1522.
(5) Jiang W, Lustig M, Larson PEZ. Concentric rings K-space trajectory for hyperpolarized 13C MR spectroscopic imaging. Magn Reson Med 2016;75:19–31.
(6) Emir UE, Burns B, Chiew M, Jezzard P, Thomas MA. Non-water-suppressed short-echo-time magnetic resonance spectroscopic imaging using a concentric ring k-space trajectory. NMR Biomed 2017. DOI 10.1002/nbm.3714.
(7) Hingerl L, Bogner W, Moser P, Považan M, Hangel G, Heckova E, Gruber S, Trattnig S, Strasser B. Density-Weighted Concentric Circle Trajectories for High Resolution Brain Magnetic Resonance Spectroscopic Imaging at 7T. Magn. Reson Med 2017. DOI 10.1002/mrm.26987.
(8) Bogner W, Gruber S, Trattnig S, Chmelik M. High-resolution mapping of human brain metabolites by free induction decay 1H MRSI at 7T. NMR Biomed 2012;25:873–882
(9) Strasser B, Chmelik M, Robinson SD, Hangel G, Gruber S, Trattnig S, Bogner W. Coil combination of multichannel MRSI data at 7 T: MUSICAL. NMR Biomed 2013;26:1796–1805.
(10) Jackson JI, Meyer CH, Nishimura DG, Macovski A. Selection of a Convolution Function for Fourier Inversion Using Gridding. IEEE Trans Med Imaging 1991;10:473–478.
(11) Pipe JG, Menon P. Sampling density compensation in MRI: Rationale and an iterative numerical solution. Magn Reson Med 1999;41:179–186.
(12) Považan M, Hangel G, Strasser B, Gruber S, Chmelik M, Trattnig S, Bogner W. Mapping of brain macromolecules and their use for spectral processing of 1H-MRSI data with an ultra-short acquisition delay at 7T. Neuroimage 2015;121:126–135.
Figures
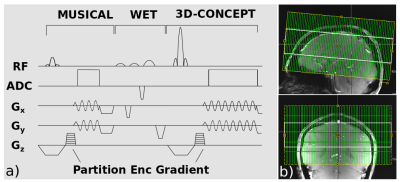
Figure 1. a) Pulse sequence diagram with MUSICAL coil combination, WET water suppression and 3D-CONCEPT. Sine and cosine modulated gradients ensure the readout of a single circle in three TRs via three temporal interleaves. Partition encoding gradients encode in the third dimension. b) Positioning of the FOV (green) and VOI (white) is illustrated.
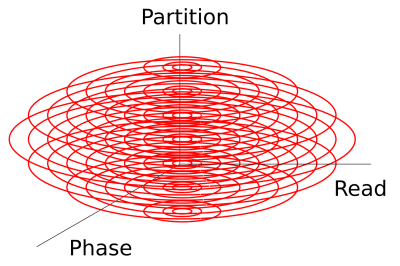
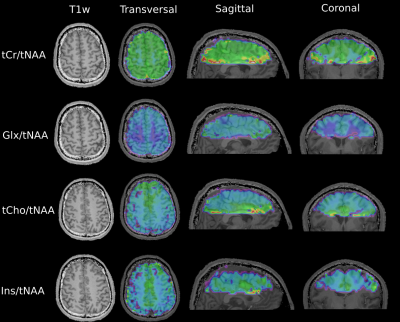
Figure 3) T1 weighted images (T1w) and transversal metabolic ratio maps of tCr=total creatine, tNAA=total N-acetyl-aspartate, Glx=glutamine + glutamate, tCho=total choline and Ins=Inositol for various slices - on the left - along with sagittal and coronal cross-sections of the 3D maps - on the right - are shown.