0605
Real-time MRI-guided endovascular model of cerebral ischemia in swine1Department of Neurology and Neurosurgery, University of Warmia and Mazury in Olsztyn, Olsztyn, Poland, 2Central Clinical Hospital of Ministry of the Interior and Administration in Warsaw, Warsaw, Poland, 3Department of Surgery and Roentgenology with the Clinic, University of Warmia and Mazury in Olsztyn, Olsztyn, Poland, 4Department of Gamete and Embryo Biology, Institute of Animal Reproduction and Food Research of the Polish Academy of Sciences, Olsztyn, Poland, 5NeuroRepair Department, Mossakowski Medical Research Centre Polish Academy of Sciences, Warsaw, Poland, 6Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 7Institute for Cell Engineering, The Johns Hopkins University School of Medicine, Baltimore, MD, United States
Synopsis
Animal models of stroke are essential for developing therapies. Rodent models of stroke are widely used but they lack clinical relevance. Endovascular models in large animals are most desired, but till now they were available in expensive and hard-to-access dogs and primates. Swine is preferred model but till now stroke modeling was through surgical craniotomy, a highly invasive procedure inflicting unrelated morbidity. Endovascular modeling was not possible due to vascular rete preventing catheter access to cerebral vessels. We circumvented this obstacle by intra-arterially injecting SPIO-labeled pro-coagulant thrombin under real-time MRI, which was instrumental to fine-tune injection to occlude cerebral arteries.
Introduction
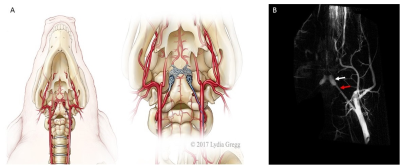
Stroke is a leading cause of serious, long-term disability.1 Rodent models play an important role in studying stroke and developing therapies; however, relying solely on rodents proved inadequate as practically all clinical trials, that were based on rodent data only, failed. STAIR2 and STEPS3 recommend testing experimental therapies of stroke in large, gyrencephalic animals prior to first-in-man studies. The endovascular approach accurately mimics clinical stroke, but until now it was available in expensive and hard to access species such as dogs and primates. The swine is a low-cost and easily available laboratory large animal, but the rete, a network of microvessels separating extracranial and intracranial circulation (Fig. 2A,B), precluded advancing intra-arterial catheter into cerebral vessels for local obstruction of cerebral blood flow. Therefore, the stroke was till now induced in pigs through highly invasive and complex surgery including craniotomy and clipping or coagulating cerebral vessels4. To address this challenge we took an advantage of our previously established interventional MRI approaches, which were proved to be excellent for monitoring intra-arterial targeting.5 We explored utility of these techniques for inducing endovascular model of cerebral ischemia in pig.
We used minimally invasive, catheter-based endovascular technique for catheter navigation under X-ray followed by MRI to guide occlusion of cerebral vessels. Real-time MRI allowed immediate visualization and continuous feedback, which were instrumental for precise administration of intraarterial thrombin until the obstruction of cerebral blood flow has been verified by contrast-enhanced DSC perfusion imaging.
Methods
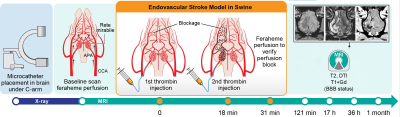
Overall experimental design is shown in Fig.1. The animal procedures were approved by local ethics committee and were performed according to ARRIVE guidelines. Juvenile, male, domestic pigs were anesthetized by propofol (3-5mg/kg/h i.v.) and sevoflurane (1 – 2,5 %). Introducer (5F, Prelude MeritMedical) was inserted into femoral artery after percutaneous puncture. Using this port, guiding catheter was navigated under C-arm guidance to common carotid artery (CCA) followed by microcatheter (APOLLO™ Medtronic) with its tip positioned in ascending pharyngeal artery (APA) proximally to the rete mirabile (Fig.2B). Continuous flow of heparinized saline was maintained in guiding catheter and microcatheter to avoid thrombotic occlusion. Animals with secured microcatheters were transferred to 3T MRI scanner (Magnetom Trio, Siemens). Under real time MRI guidance thrombin solution (Biomed, Poland) mixed with feraheme (1:20) was injected intra-arterially as two boluses (80 Units and 320 Units respectively) with 20 minutes interspace. MRI protocol included dynamic GE-EPI for assessment of trans-catheter cerebral perfusion, GE-EPI for monitoring thrombin-mediated blood clotting as well as SWI, diffusion, T2w and T1w with contrast. MRI follow up was performed one day, two days and one month post stroke induction. Images were analyzed using AMIRA6.4 (Thermo Fisher Scientific – FEI).Results
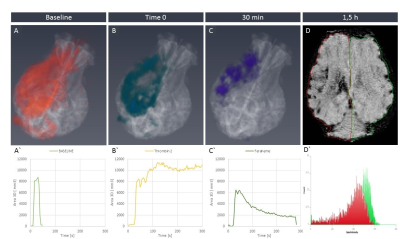
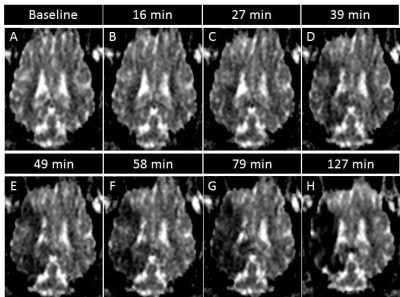
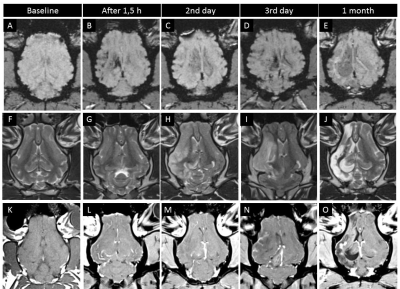
Baseline feraheme-enhanced perfusion MR scans showed brain territory supplied by the catheter infusion (3D reconstruction of perfused area is shown in Fig.3A, rapid clearance of the contrast shown in graph 3A’). Thrombin injection visualized in real-time on dynamic GE-EPI scans resulted in only minor vascular blockage after first bolus but second infusion was effective with extensive retention of hypointensity shown as 3D reconstruction Fig.3B and dynamically quantified in Fig.3B’. SWI scan confirmed retention of hypointensities indicating vascular blockage (Fig.3D). Perfusion was visibly smaller after thrombin as shown with another feraheme-enhanced perfusion scan (8,754.4 mm3 before thrombin and 6,484.59 mm3 after clot formation; Fig.3C). Notably, there was dramatic difference in clearance of feraheme with rapid clearance at baseline lasting ~18 sec (Fig.3A’) and sluggish clearance after thrombin ~250 sec (Fig.3C`). Diffusion weighted MRI detected first evidence of ischemic damage 27 minutes post thrombin as hypointense region on ADC map (Fig.4C), with subsequent intensification of pathological signal. T2w scan 17 hours post stroke induction confirmed ischemia (Fig.5H). Assessment of BBB status with T1+Gd showed no enhancement over initial 17 hours and disruption was observed in the infarcted cortex 36 hours after procedure (Fig.5N). One-month follow-up revealed BBB opening within white matter (Fig.5O). Lesion resulted in marked neurological deficits in contralateral limbs primarily manifested as severe hind limb paresis, however the animal was ambulatory and did not required an intensive care.Conclusion
Our study has demonstrated feasibility of using endovascular route to induce ischemic stroke in pig. Most importantly, real-time MRI was instrumental to monitor and confirm formation of thrombin-induced clot and resulting blockade of cerebral perfusion with subsequent brain infarct. Our method produced cerebral ischemia in relatively large volume, which covers majority of MCA supply territory. Overall, we have established a new model of ischemic stroke in pigs characterized by high clinical relevance.Acknowledgements
Funding: NCBIR EXPLOREME grant (STRATEGMED1/235773/19/NCBR/2016)References
1 Writing Group, M. et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 133, e38-360, doi:10.1161/CIR.0000000000000350 (2016).
2 Fisher, M. et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke 40, 2244-2250, doi:10.1161/STROKEAHA.108.541128 (2009).
3 Savitz, S. I. et al. Stem Cell Therapy as an Emerging Paradigm for Stroke (STEPS) II. Stroke 42, 825-829, doi:10.1161/STROKEAHA.110.601914 (2011).
4 Arikan, F. et al. Malignant infarction of the middle cerebral artery in a porcine model. A pilot study. PLoS One 12, e0172637, doi:10.1371/journal.pone.0172637 (2017).
5 Janowski, M., Walczak, P. & Pearl, M. S. Predicting and optimizing the territory of blood-brain barrier opening by superselective intra-arterial cerebral infusion under dynamic susceptibility contrast MRI guidance. J Cereb Blood Flow Metab 36, 569-575, doi:10.1177/0271678X15615875 (2016).
Figures