0603
Real-time MRI endoscopy at up to 10 frames/sec1Electrical and Computer Engineering, Johns Hopkins University, Baltimore, MD, United States, 2Russell H. Morgan Dept. of Radiology, Johns Hopkins University, Baltimore, MD, United States, 3Biomedizinishe NMR Forschungs GmbH, Max-Plank-Institut fur biophysikalische Chemie, Gottingen, Germany
Synopsis
Mininally-invasive intravascular MRI at 3T and above is capable of providing high resolution imaging from within blood vessels and identifying atherosclerosis using miniaturized detectors ~2mm in diameter. Endoscopic MRI is a technique that employs the miniature probe itself to localize the MRI signal to a sensitive disk, and provide images from the view-point of the probe itself. At 3T, acquisition speed has been limited to 2 frames/sec at 300μm resolution, which although fast, is not truly real-time. Here we report a truly real-time MRI endoscope with fully integrated real-time continuous MRI visualization at up to 10 frames/sec.
Purpose
Minimally invasive intravascular MRI employing miniaturized MRI coils can provide high-resolution imaging and soft tissue contrast for characterizing normal vessel wall and different stages of atherosclerosis not detectable by conventional X-ray catheterization, without ionizing radiation(1,2). MRI endoscopy is a further advance that utilizes the highly-localized sensitivity profile of the miniature coil, in combination with adiabatic transmission to provide imaging that is intrinsically locked to a ‘sensitive disk’ at the end of the probe that moves with it. Advancing the device into a blood vessel or orifice thus provides an endoscopic view–at a spatial resolution that is not possible with conventional external MRI.
To date, MRI endoscopy has been limited to 2 frames/sec at 300µm resolution in clinical 3T MRI scanners(1). The speed was limited by conventional scan sequences, the inability to use sensitivity encoding (SENSE) methods with the single-channel device, and the reconstruction time and limited acceleration achievable with existing compression methods(3). Consequently, the truly real-time image visualization that is essential for interventional MRI has not been realized. Researchers at Goettingen Max-Planck-Institut (MPI) developed a system comprised of under-sampled radial MRI sequences and reconstruction algorithms employing high-speed graphics processing units (GPUs) enabling truly real-time MRI on a 3T clinical scanner(4). We have adapted this technology to perform MRI endoscopy at up to 10 frames/sec, and report initial results on human vessel specimens at 3T.
Methods
An MPI GPU based acceleration unit was fitted to a standard clinical Siemens clinical 3T Prisma MRI scanner via a single high-speed Ethernet cable and configured to allow continuous real-time image display. MRI is accelerated using highly-undersampled radial acquisition, reconstructed with the temporally-regularized nonlinear inversion (NLINV) algorithm(4-6) which jointly estimates image and coil sensitivity. Online image reconstruction is realized using a highly parallelized version of the NLINV algorithm(7) and a ‘bypass’ computer (‘sysGen/TYAN Octuple-GPU’, Sysgen, Bremen) equipped with 8 GPUs (GeForce GTX 580, TITAN, Nvidia, Santa Clara). The MRI endoscopy sequence was implemented by omitting the slice-selective gradients of an undersampled radial FISP sequence, and replacing the excitation by adiabatic B1-independent rotation (BIR4) pulses(1,8).
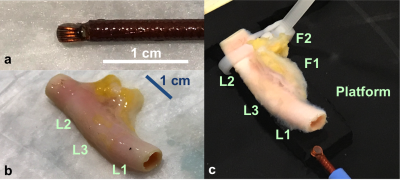
An endoscopic loop MRI coil (Fig.1a) was fabricated as a 4.5-turn 3mm diameter solenoid and tuned with a 56pF micro-capacitor for a 3T Siemens PRISMA MRI scanner, and mounted on a flexible cable terminated with an integrated bazooka balun to provide a high impedance at the coil to suppress cable signals(8). The loop coil was used in a transmit/receive mode, and interfaced to two ganged preamplifiers on the scanner. Fresh human carotid artery specimens obtained from our pathology department (Fig.1b) were mounted in a tank (Fig.1c), and the MRI endoscope advanced through their lumens during MRI. Sagittal and coronal scout images (FLASH, resolution 0.8mm) were acquired to determine coil sensitivity profiles.
MRI endoscopy was performed with continuous application of the BIR4-modified true-FISP real-time MPI sequence (TR/TE = 12/6ms, 90° BIR-4 pulse, FOV 64mm, 300µm resolution, 5-10 frames/sec). Data were acquired as MRI cine streams at acquisition rates of 10 frames/sec (9 radial projections) and 5 frames/sec (17 radial projections). As validation, conventional non-accelerated high-resolution MRI endoscopy was performed(1) at corresponding locations along the vessel (True-FISP, TR/TE = 23/11ms, 90° BIR-4 pulse, 100µm resolution, FOV 20mm, acquisition time 4.5s).
Results
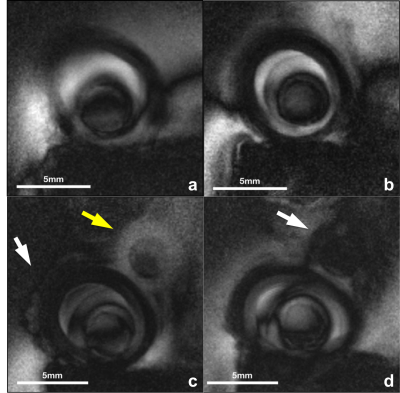
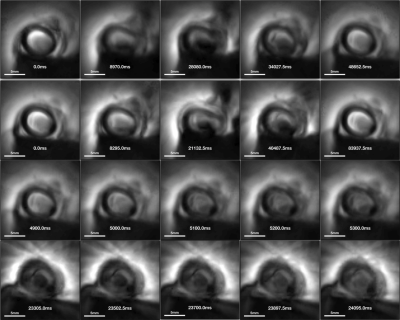
Coil sensitivity was successfully confined to <5mm at the coil end, permitting its use as an endoscope without slice selection (1,8). High-resolution (100µm) images obtained with the conventional MRI endoscopy sequence at four locations are exemplified in Fig.2. The peri-vascular fat increases as the coil moves from near L1 to L2 (Fig.3a-d), consistent with Fig.1c and compression of the vessel by the tape at the end(Fig. 3d). Cine MRI frames (Fig.3) correlate with corresponding high-resolution MRI. Rows 1 and 2 of Fig.3 are snapshots acquired at 10Fr/s and 5Fr/s, traversing from left to right frames near L1, L3, L2, L3, and L1. Consecutive frames in rows 3 and 4 illustrate the difference between the two acquisition rates: with some radial reconstruction artifact appearing more pronounced at 5Fr/s perhaps due to the moving probe.Discussion
This work demonstrates for the first time, true real-time MRI endoscopy at 10 frames/sec and 300µm resolution on a clinical 3T scanner. Such performance is presently unobtainable with external coils or indeed other means. High speed MRI endoscopy offers a new ’scope for accessing the body at high-resolution with MRI’s soft-tissue sensitivity, ability to see through vessel walls, calcifications, blood etc, as compared to conventional optical and ultrasound endoscopic approaches.Acknowledgements
Supported by NIH grant R01007829, and a grant by Siemens Medical Systems.References
1. Sathyanarayana S, Schär M, Kraitchman DL, and Bottomley PA. Towards Real-Time Intravascular Endoscopic Magnetic Resonance Imaging. JACC: Cardiovascular Imaging. 2010;3(11):1158-1165.
2. Wang G, Zhang Y, Hegde SS, Bottomley PA. High-resolution and accelerated multi- parametric mapping with automated characterization of vessel disease using intravascular MRI. J Cardiovasc Magn Reson 2017; DOI 10.1186/s12968-017-0399-6.
3. Hegde SS, Zhang Y, Bottomley PA. Acceleration and motion-correction techniques for high-resolution intravascular MRI. Magn Reson Med 2015;74:452–61.
4. Uecker M, Zhang S, Voit D, Karaus A, Merboldt KD, Frahm J. Real-time MRI at a resolution of 20 ms. NMR Biomed 2010; 23: 986-994.
5. Uecker M, Hohage T, Block KT, Frahm J. Image reconstruction by regularized nonlinear inversion – Joint estimation of coil sensitivities and image content. Magn Reson Med 2008; 60: 674-682.
6. Uecker M, Zhang S, Frahm J. Nonlinear inverse reconstruction for real-time MRI of the human heart using undersampled radial FLASH. Magn Reson Med 2010; 63: 1456-1462.
7. Schaetz S, Uecker M. A multi-GPU programming library for real-time applications. Lect Notes Comput Sci 2012; 7439: 114-128.
8. Sathyanarayana S, Bottomley PA. MRI endoscopy using intrinsically localized probes. Med Phys. 2009;36:908-19.
Figures