0601
Fully MR-Guided Implantation of a Bioresorbable Scaffold in the Left Coronary Artery of a Pig at 3T1Dept. of Radiology, Medical Physics, Medical Center University of Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Germany, 2Dept. of Cardiology and Angiology I, University Heart Center, Freiburg, Germany, 3MaRVis Interventional GmbH, Frechen, Germany
Synopsis
MR-guided cardiovascular interventions do not use ionizing radiation, provide excellent soft tissue contrast, and enable functional measurements. The feasibility of MR-guided percutaneous coronary intervention has been demonstrated in animal trials using metallic stents. Recently, bio-resorbable vascular scaffolds have been introduced for stenting of coronary arteries. These scaffolds enable artifact-free MR imaging of the coronary artery segment that the result of the intervention can be readily assessed via MR imaging. We show the first fully MR-guided PCI with a bioresorbable scaffold in the LCA of a pig at 3T using dedicated active guiding catheters, an MR-safe guidewire and BVS delivery system.
Introduction
MR-guided cardiovascular interventions do not use ionizing radiation, provide excellent soft tissue contrast, offer arbitrary slice orientations and enable functional measurements as well as tissue characterization of the myocardium. Although MR-guided catheterizations of the heart are already performed in humans1, percutaneous coronary interventions (PCI) are still challenging to perform under MR guidance due to limited spatial and temporal resolution. However, animal trials at 1.5T demonstrated the feasibility of MR-guided PCI2,3, and recent developments in coronary MRI4 as well as real-time imaging techniques5 may help to overcome current limitations. Furthermore, dedicated active guiding catheters and MR-visible guidewires were developed to enable the engagement of the coronary artery under MR guidance6,7.
Recently, bio-resorbable vascular scaffolds (BVS) have been introduced for stenting of coronary arteries. BVS are free of metal and thus enable artifact-free MR imaging of the scaffolded coronary artery segment8 which is desirable for MR-guided PCI as the result of the intervention can be readily assessed via MR imaging.
In this study, we show the first MR-guided implantation of a BVS in a pig at 3T using an active guiding catheter and an MR-safe guidewire and BVS delivery system.
Methods
For engagement of the left coronary artery (LCA), an 8F catheter with non-metallic braiding and Tiger tip shape was designed as a guiding catheter. The distal end was equipped with a 2cm-long single-loop coil to allow for visualization of the catheter tip during real-time MR imaging. The coil was connected to one receive channel of the MR system via a coaxial cable embedded in the catheter’s wall and a tune/match circuit with variable attenuation9.
For BVS implantation a non-metallic BVS delivery system (Abbott Vascular, Wetzlar, Germany) was used in combination with an MR-safe 0.014” guidewire (MaRVis Interventional GmbH, Frechen, Germany) which was doped with iron microparticles for MR visibility.
The MR-guided PCI was performed in a female Göttingen minipig on a clinical 3T MR system (Siemens PRISMA). A 10F sheath was placed in the right femoral artery for arterial access. The pig was mechanically ventilated and general anesthesia was maintained by isoflurane inhalation. Initial localization of the coronary arteries was done via a whole-heart 3D navigator-gated FLASH sequence (fat saturation, T2 preparation, TE/TR: 1.6/3.5 ms, FA: 16°, FoV: 2822x102 mm³, matrix: 1762x64, TET2prep: 40 ms, R = 2). A radial bSSFP sequence was used for real-time imaging during the engagement of the LCA with the active guiding catheter (TE/TR: 1.4/2.8 ms, spokes: 105, FA: 40°, FoV: 2752x7 mm³, matrix: 160x160, fat saturation).
Once the catheter tip was in the LCA, contrast-enhanced MR angiography images were acquired during infusion of a 5 ml bolus (5% Gd-DTPA solution) through the guiding catheter. Contrast enhancement in the myocardium was imaged using an ECG-triggered FLASH acquisition with saturation recovery in short-axis view (TE/TR: 1.1/2.2 ms, TSR: 102 ms, FA: 8°, FoV: 225x300 mm³, matrix: 120x160, R = 2). Then, the guidewire was advanced into the LCA followed by the BVS delivery catheter. The balloon was dilated to place an 18mm-long BVS (Ø 3 mm) in the artery. For visualization of the balloon, a 1% Gd-DTPA solution was used in combination with the real-time radial bSSFP sequence as before with an additional saturation pulse (TSR = 170 ms).
Results
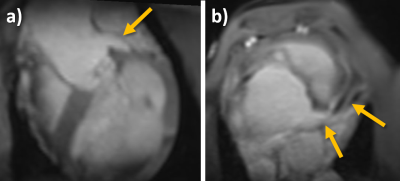
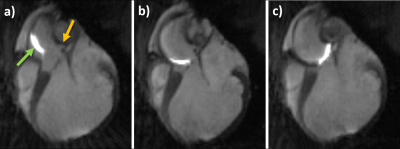
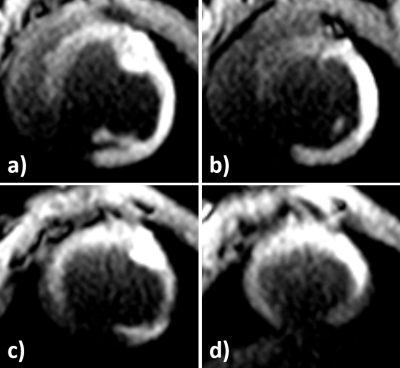
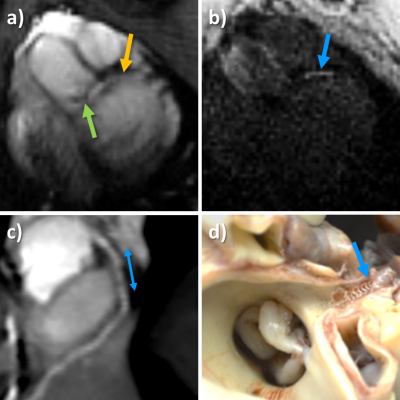
Planar reformats of the left ostium and LCA are seen in Figure 1. These images are acquired before the intervention and are used as reference for the real-time imaging. Figure 2 shows real-time images of the successful engagement of the LCA with the active guiding catheter. The catheter tip and its curvature as well as the coronary ostium are well visible. The perfusion measurement performed after the engagement clearly depicts the myocardial area supplied by the LCA (Figure 3). The BVS was implanted in the left main stem at a distance of about 1 cm from the ostium. Real-time imaging with saturation recovery shows the dilated balloon (Fig 4b) in the main stem (Fig 4a). 3D imaging of the LCA after BVS implantation shows the open scaffolded lumen without any artifacts coming from the BVS (Fig 4c). The successful implantation of the BVS was verified by analysis of the explanted heart (Fig 4d).Conclusion
This study demonstrates the feasibility of the fully MR-guided PCI with a non-metallic bioresorbable scaffold in the LCA of a pig at 3T. The BVS was implanted in a proximal part of the LCA which is less difficult to image in real-time than more distal parts of the coronary arteries. Further work will thus aim for the improvement of real time imaging techniques and visualization of the guidewire and the BVS delivery catheter to enable BVS implantations in more distal parts of the coronary arteries.Acknowledgements
Grant support by the Deutsche Forschungsgemeinschaft (DFG) under grant number BO 3025/2-2 and the ESC grant for medical research innovation is gratefully acknowledged.References
1. Ratnayaka K, Kanter JP, Faranesh AZ, et al. Radiation-free CMR diagnostic heart catheterization in children. J. Cardiov. Magn. Reson. 2017;19(1):65
2. Spuentrup E, Ruebben A, Schaeffter T, et al. Magnetic Resonance–Guided Coronary Artery Stent Placement in a Swine Model. Circulation 2002;105(7):874–879
3. Omary RA., Green JD, Schirf BE. Real-Time Magnetic Resonance Imaging-Guided Coronary Catheterization in Swine. Circulation 2003;107(21):2656–2659.
4. Ginami G, Coppo S, Feng L, et al. Cardiac and Respiratory Motion-Resolved Free-Running Whole-Heart Coronary MRA of Patients Using 5D XD-GRASP Reconstruction. Proc. Intl. Soc. Mag. Reson. Med. 2016
5. Zhang S, Uecker M, Voit D, et al. Real-Time Cardiovascular Magnetic Resonance at High Temporal Resolution: Radial FLASH with Nonlinear Inverse Reconstruction. J. Cardiov. Magn. Reson. 2010;12:39
6. Bock M, Wacker FK. MR-Guided Intravascular Interventions: Techniques and Applications. J. of Magn. Reson. Imaging 2008; 27(2):326–338
7. Reiss S, Özen AC, Lottner T. et al. Active catheter tracking for MR-Guided Percutaneous Coronary Intervention at 3T: Initial Results in a Pig Model, Proc. Intl. Soc. Mag. Reson. Med. 25 (2017)
8. Reiss S, Krafft AJ, Zehender M, et al. Magnetic Resonance Imaging of Bioresorbable Vascular Scaffolds. Circ Cardiovasc Interv 2015;8(4):e002388
9. Özen AC, Lottner L, Reiss S, et al. Operator Controlled Illumination of Active Catheter Tips using a Variable Attenuator. Proc. Intl. Soc. Mag. Reson. Med. 25 (2017)
Figures