0595
Intra-operative acquisition of sensorimotor fMRI during glioma resection: evaluation of feasibility and clinical applicability.1Neuroradiological Academic Unit, UCL Institute of Neurology, London, United Kingdom, 2Psychology and Language Sciences, Birkbeck-UCL Centre for Neuroimaging, London, United Kingdom, 3Lysholm Department of Neuroradiology, National Hospital for Neurology and Neurosurgery, London, United Kingdom, 4Medical Physics and Biomedical Engineering, University College London Hospital, London, United Kingdom, 5Wellcome Trust Centre for Neuroimaging, UCL Institute of Neurology, London, United Kingdom, 6Department of Neurosurgery, National Hospital for Neurology and Neurosurgery, London, United Kingdom, 7Department of Neuroanaesthesia, National Hospital for Neurology and Neurosurgery, London, United Kingdom, 8Department of Neurophysics, Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany
Synopsis
Intra-operative fMRI has the potential to improve neurosurgical outcomes and we have previously shown that the task-related BOLD signal can be acquired under general anaesthesia. Our next goal was to acquire fMRI intra-operatively with the skull open.
In 12 patients, we performed 24 acquisitions of a passive sensorimotor paradigm during the resection of their brain tumour. The fMRI data were evaluated by neuroradiologists, assessing its applicability for the provision of a clinical report on the location of sensorimotor activation to the neurosurgeon. 17/24 acquisitions were scored as useful.
We conclude that intra-operative fMRI is feasible and produces clinically useful data.
Purpose
Accurate localisation of functionally important brain areas intra-operatively is a prerequisite for the safe resection of brain tumours. With the increasing availability of intra-operative MRI, a potential solution is to acquire fMRI intra-operatively during the resection itself. We have previously demonstrated that the task-related BOLD signal can be accurately and reproducibly detected under deep unconsciousness resulting from neurosurgical anaesthesia1.
The next step was to perform a passive sensorimotor paradigm during the tumour resection procedure with the patients unconscious, the skull open and brain tissue exposed. Our aim was to 1) determine if task-related BOLD activation can be acquired intra-operatively under the conditions associated with craniotomy and tumour resection, and if so 2) to assess the potential clinical usefulness of the activation data.
Methods
With ethical committee approval, 12 adult patients with gliomas in a cerebral hemisphere (5 left and 7 right hemispheric lesions) underwent neurosurgical resection in an intra-operative MRI unit with a 1.5T Siemens ESPREE system using a dedicated 8-channel receive-only surgical head coil. A passive finger flexion sensorimotor fMRI paradigm2 was performed during the surgery under deep anaesthesia with the craniotomy bone flapped removed and with tumour resection ongoing. The paradigm was performed on the right and left hands to assess activation in both the exposed (side of surgery) and unexposed cerebral hemispheres. The clinical depth of anaesthesia was measured at the start of surgery using a processed EEG-based system and the anaesthesia (sevoflurane) concentration was recorded.
fMRI used a gradient-echo EPI sequence with voxel size of 3 x 3 x 3 mm3 (TR/TE/flip angle = 3100ms/40ms/90°), field of view = 192mm x 192mm, 104 volumes of a 42 slice whole brain acquisition. For anatomical reference, 3D MPRAGE T1-weighted structural images were acquired. Preprocessing, including normalisation and the statistical analysis of the fMRI data was performed in SPM12. The data was analysed using a subject specific fixed effect analysis using the GLM3 and reviewed using variable statistical thresholding ranging from p=0.001 to p=0.05 (uncorrected).
A total of 24 data sets (12 for each hand) were reviewed by two neuroradiologists to assess 1) if there was BOLD activation in the expected somatotopic region of the sensorimotor cortex and 2) whether the data was sufficient that a clinical neuroradiological report could be provided to the neurosurgeon as to the location of sensorimotor activation detected intra-operatively. Each data set was scored as being either a) useful or b) suboptimal. The possible influence of the concentration of the anaesthesia, sevoflurane, on these results was then explored with a Mann-Whitney U test (SPSS).
Results
In 17/24 acquisitions, BOLD activation was obtained within the somatotopic region of the sensorimotor cortex (Table 1a and 1b) and the data were scored to be clinically useful (examples shown in Figure 1). In 7/24 acquisitions, the data were scored to be suboptimal either because the clusters were too small (n=3) or could not be identified (n=4) at a threshold of p=0.05 (Figure 2). 6/7 of the suboptimal acquisitions came from the same three patients.
The median sevoflurane concentration in each of the groups scored as being useful and suboptimal was 1.6 and 2.1 in the exposed hemisphere and 1.7 and 2.3 in the unexposed hemisphere (Figure 2), though these differences were not significantly different (p=0.11 (exposed) and p=0.064 (unexposed)).
Discussion
The first aim of this study was to determine if task-related BOLD activation could be detected in 12 anaesthetised neurosurgical patients intra-operatively during the resection of their brain tumour and this was demonstrated in 20/24 (83%) of acquisitions. The second aim was to assess whether the data was sufficient that a clinical neuroradiological report could be provided to the neurosurgeon as to the location of intra-operative sensorimotor fMRI activation and this was found in 17/24 (71%) of acquisitions.
We have previously shown that sevoflurane anaesthesia significantly reduces the BOLD signal amplitude1. Though no significant difference was demonstrated in the sevoflurane concentration between the data sets scored as a) useful vs b) suboptimal, individual patient differences in sensitivity to anaesthesia and other physiological factors may have contributed to those acquisitions scored as suboptimal.
Conclusion
In a cohort of neurosurgical patients, we have demonstrated that the task-related BOLD signal can be reproducibly acquired intra-operatively during tumour resection and in the majority of cases, the data can prove useful in providing a neuroradiological report to the neurosurgeon as to the location of intra-operative sensorimotor fMRI activation.
These results provide strong evidence supporting the feasibility and indicating the possible role of intra-operative fMRI.
Acknowledgements
This research was funded by a grant provided by the NIHR UCL/UCLH Biomedical Research Centre.References
1. Yamamoto AK, Magerkurth J, Mancini L, White MJ , Miserocchi A, McEvoy AW, et al. Acquisition of sensorimotor fMRI under general anaesthesia in neurosurgical patients: evaluation of the effect of anaesthesia on the BOLD response. 1241 Proc. Intl. Soc. Mag. Reson. Med. 25 (2017)
2. Magerkurth J, Mancini L, Penny W, Flandin G, Ashburner J, Micallef C, et al. Objective Bayesian fMRI analysis-a pilot study in different clinical environments. Front Neurosci 9:1–17 (2015)
3. Friston, K.J., Frith, C.D., Turner, R., Frackowiak, R.S. Characterizing evoked hemodynamics with fMRI. Neuroimage 2, 157–65 (1995)
Figures
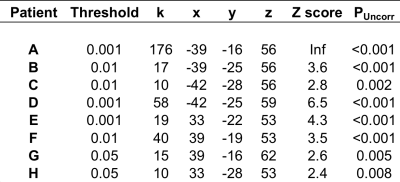
Table 1a
The statistical results for the 17 fMRI acquisitions scored as being useful for provision of a clinical neuroradiological report are listed according to whether the data was acquired in the exposed (table 1a) or unexposed (table 1b) hemisphere at the time of tumour resection. Threshold = statistical height threshold selected by the neuroradiologist, k=cluster size, x y z =MNI co-ordinates for peak voxel activation, Z score and P value for peak voxel activation.
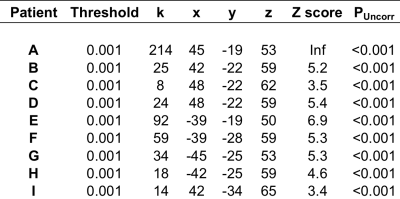
Table 1b
The statistical results for the 17 fMRI acquisitions scored as being useful for provision of a clinical neuroradiological report are listed according to whether the data was acquired in the exposed (table 1a) or unexposed (table 1b) hemisphere at the time of tumour resection. Threshold = statistical height threshold selected by the neuroradiologist, k=cluster size, x y z =MNI co-ordinates for peak voxel activation, Z score and P value for peak voxel activation.
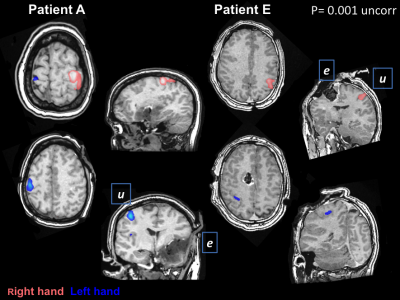
Figure 1
Illustrates examples of the left and right hand fMRI acquisitions for two patients, A and E, both scored as being clinically useful. Images are shown in radiological convention (left of patient shown on left side of image). The fMRI clusters are colour coded according to whether activation was elicited by movement of the right or left hand. Patient A has a tumour in the left cerebrum so the left hemisphere was exposed, patient E has a tumour in the right cerebrum (right hemisphere exposed).
e = exposed hemisphere, u = unexposed hemisphere.
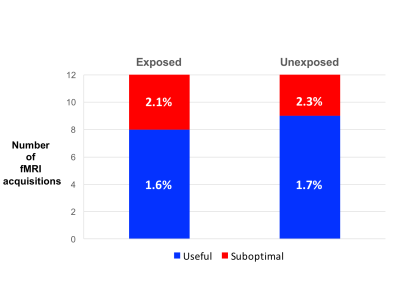
Figure 2
Illustrates the results for the 24 fMRI acquisitions as to whether they were scored as being a) useful (blue) or suboptimal (red) . Exposed indicates the fMRI was acquired in the cerebral hemisphere exposed at the time of surgery, unexposed indicates activation was acquired in the contra-lateral hemisphere.
% is the group median end-tidal sevoflurane concentration.