0593
Finding the baby in the bath water – evidence for task-specific changes in resting state functional connectivity evoked by training1National Institute of Mental Health, Bethesda, MD, United States
Synopsis
Resting-state fMRI (rsFMRI) has been used for studying training-related changes in brain function during the offline period of skill learning. However, the lack of experimental control during “rest” makes it difficult to separate the impact of training from technical artifacts and experimental confounds like time-of-day (TOD) related changes in MRI signal. Here, by using multiple tasks (rest, visuo-spatial training, motor sequence training), we mapped out the spatial topography of changes in rsFC evoked by TOD and by training. Our findings suggest that task-specific changes in rsFC due to visuo-spatial and motor-sequence learning are dissociable from changes due to TOD.
Purpose
To examine whether training in a visuo-spatial and motor-sequence learning task evoke task-specific changes in rsFC that are dissociable from time-of-day related changes.Introduction
Resting-state fMRI (rsFMRI), which offers a measure of functional connectivity (rsFC) between brain regions during rest, has been used for studying training-related changes in brain function during the offline period of skill learning1,2,3,4,5,6. However, the lack of experimental control during “rest” makes it difficult to separate the impact of training from technical artifacts and experimental confounds. For example, it is difficult to infer whether any changes in rsFC evoked by skill training between two scans within a day, are specific to the task, or if they are due to diurnal fluctuations in MRI signal7,8,9. Here, we sought to elucidate whether training in a visuo-spatial and motor-sequence learning task evoke task-specific changes in rsFC that are dissociable from time-of-day (TOD) related changes.Methods
A group of 19 healthy young adults (mean age: 25.8 years, 10 female) were scanned during four visits (each visit scheduled one week apart) on a 3 Tesla GE MR750 scanner using a GE 32-channel head coil. Each visit included scan sessions in the AM and PM. On Visits 1 and 4, participants received no training, while during visits 2 and 3, between the AM and PM scans, participants received 90 minutes of training in a visuo-spatial task and a motor sequence learning task respectively. Behavioral measures included improvement in: visuo-spatial memory, response time for executing motor sequence immediately after training, and 1 day after training (i.e. overnight consolidation). Two resting state scans were acquired during each session, along with respiration data [scan details: sequence: 2D-EPI, 80 × 80 matrix slice, resolution: 3 x 3 x3 mm; TR: 2000 ms; TE: 27 ms; flip angle: 770; acquisition time: 5 min; instruction: fixate on a cross]. The rsFMRI data were despiked, detrended, bandpass filtered and the time series for nuisance factors (e.g. respiration, motion) were regressed out using AFNI10. Given the nature of the training tasks we focused on the hippocampal network (defined anatomically) and the sensorimotor network (defined using an independent functional localizer). The mean time series was extracted separately from each participant and each rest session to generate whole brain correlation maps (Pearson’s r) for the two networks of interest. To test for differences in connectivity patterns across visits, the seed-based connectivity maps were submitted to a linear mixed effects model (3dLME11) with Hemisphere (left/right), Visit (Visits 1-4), and TOD (AM/PM, equivalent to Pre/Post training in the context of Visits 2 and 3) included as factors for both networks (hippocampus and sensorimotor) separately. In addition, we also tested for correlations between rsFC changes evoked by training and improvements in performance using 3dLME with TOD as a factor, and performance improvement as a covariate of interest.Results
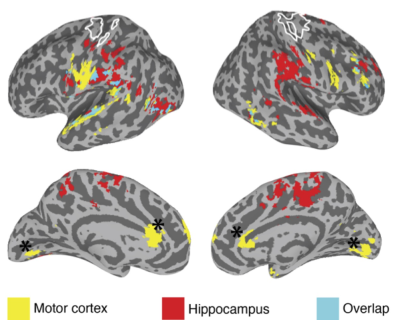
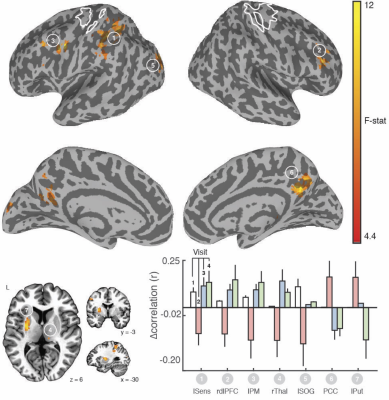
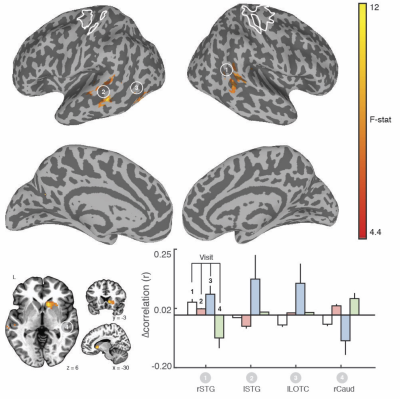
We found clear evidence that the visuo-spatial and motor sequence training protocols induced a significant change in behavioral performance. With regard to rsFC changes, we found evidence for TOD-related fluctuations in both networks (Fig. 1). Controlling for the effect of TOD (i.e. TOD x Visit interaction), reveaa spatially distinct pattern of changes in rsFC in the hippocampal network that was specific to visuospatial learning (Fig. 2). Similar analysis on the sensorimotor network revealed changes in rsFC that were specific to motor-sequence training and spatially distinct from the regions showing modulations in the hippocampal network and modulations due to TOD (Fig. 3). Mapping the brain areas that show a strong correlation between behavioral measures and change in rsFC within the two networks revealed a spatially distinct pattern of brain areas for visuo-spatial and motor sequence learning.Discussion
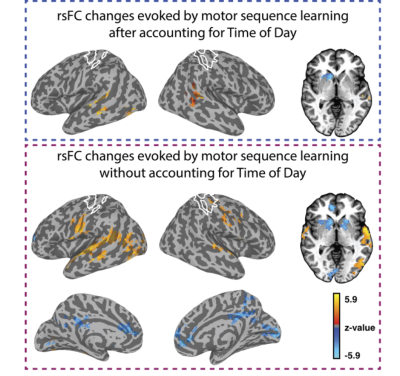
Our study provides evidence that even in the absence of any experimental manipulation, significant changes in rsFC can be detected between two identical rsFMRI scans performed a few hours apart. However, our findings also suggest that task-specific changes in rsFC due to visuo-spatial and motor-sequence learning that are dissociable from changes due to TOD, can be detected. In addition, our results suggest that changes in rsFC after training were predictive of performance after short-term learning and overnight consolidation, but the spatial topography differed from those showing modulation by training. Overall, our study suggests that rsFMRI can detect training-specific changes. However, because changes in rsFC due to factors such as time-of-day can confound meaningful interpretation of the results (Fig. 4), careful experimental design, including both rest and active controls is necessary.Acknowledgements
This work was supported by the Intramural Research Programs of the National Institute of Mental Health (NCT00001360, 93-M-0170). Salary support for CT was partly provided by funding from the Department of Defense in the Center for Neuroscience and Regenerative Medicine. We thank Beth Aguila and Marcie King for assistance with data acquisition. This study used the high-performance computational capabilities of the Biowulf Linux cluster at the National Institutes of Health, Bethesda, Maryland (biowulf.nih.gov).References
1. Albert, N. B., Robertson, E. M., & Miall, R. C. (2009). The resting human brain and motor learning. Current Biology, 19(12), 1023–1027. doi:10.1016/j.cub.2009.04.028
2. Ma, L., Narayana, S., Robin, D. A., Fox, P. T., & Xiong, J. (2011). Changes occur in resting state network of motor system during 4 weeks of motor skill learning. Neuroimage, 58(1), 226–233. doi:10.1016/j.neuroimage.2011.06.014
3. Taubert, M., Lohmann, G., Margulies, D. S., Villringer, A., & Ragert, P. (2011). Long-term effects of motor training on resting-state networks and underlying brain structure. Neuroimage, 57(4), 1492–1498.
4. Sami, S., Miall, R.C., 2013. Graph network analysis of immediate motor-learning induced changes in resting state BOLD. Front Hum Neurosci 7, 166. doi:10.3389/fnhum.2013.00166
5. Sami, S., Robertson, E.M., Miall, R.C., 2014. The time course of task-specific memory consolidation effects in resting state networks. J Neurosci 34, 3982–3992. doi:10.1523/JNEUROSCI.4341-13.2014
6. Daselaar, S.M., Huijbers, W., de Jonge, M., Goltstein, P.M., Pennartz, C.M.A., 2010. Experience-dependent alterations in conscious resting state activity following perceptuomotor learning. Neurobiol Learn Mem 93, 422–427. doi:10.1016/j.nlm.2009.12.009
7. Hodkinson, D. J. et al. Circadian and homeostatic modulation of functional connectivity and regional cerebral blood flow in humans under normal entrained conditions. Journal of Cerebral Blood Flow & Metabolism 34, 1493-1499 (2014).
8. Trefler, A. et al., Impact of time-of-day on brain morphometric measures derived from T 1-weighted magnetic resonance imaging. NeuroImage 133, 41-52 (2016).
9. Jiang, C., Yi, L., Su, S., Shi, C., Long, X., Xie, G., Zhang, L., 2016. Diurnal Variations in Neural Activity of Healthy Human Brain Decoded with Resting-State Blood Oxygen Level Dependent fMRI. Front Hum Neurosci 10, 634. doi:10.3389/fnhum.2016.00634
10. Cox, R.W., 1996. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29, 162–173. doi:10.1006/cbmr.1996.0014
11. Chen, G., Saad, Z.S., Britton, J.C., Pine, D.S., Cox, R.W., 2013. Linear mixed-effects modeling approach to FMRI group analysis. Neuroimage 73, 176–190. doi:10.1016/j.neuroimage.2013.01.047
Figures