0590
Resting-state functional connectivity alterations in adolescents with fetal alcohol spectrum disorders1MRC/UCT Medical Imaging Research Unit, Division of Biomedical Engineering, University of Cape Town, Cape Town, South Africa, 2Department of Human Biology, University of Cape Town, Cape Town, South Africa, 3Department of Psychiatry and Behavioral Neurosciences, Wayne State University School of Medicine, Detroit, MI, United States, 4Department of Psychiatry and Mental Health, University of Cape Town, Cape Town, South Africa
Synopsis
In a study of children with fetal alcohol spectrum disorders (FASD) and community controls assessed at age 11 years, alcohol-exposed children showed
Introduction
Resting-state functional MRI (rs-fMRI), which assesses functional connectivity (FC) between spatially distinct brain regions while a subject is not performing any explicit task [Biswal et al., 1995], is a sensitive marker of alterations in brain development [Thomason et al., 2011; de Bie et al., 2012; Supekar et al., 2010] and disease [Greicius et al., 2008]. Fetal alcohol spectrum disorders (FASD) encompass the range of cognitive, behavioral, and neurological impairments caused by prenatal alcohol exposure (PAE). Previously, we found dose-dependent FC reductions in 5 gray matter (GM) regions within 5 networks in 11-year old children with FASD [Fan et al., 2017]. The aim of this study was to examine RSFC alterations in these individuals during adolescence.Methods
Participants were 56 Cape Coloured (mixed ancestry) adolescents (mean age ± std dev = 15.8±0.9 yr; 20 fetal alcohol syndrome (FAS)/partial FAS (PFAS), 13 heavily exposed (HE) nonsyndromal children and 23 non- or minimally exposed controls) from the Cape Town Longitudinal Cohort [Jacobson et al., 2008], whose mothers were recruited during pregnancy from a community where heavy drinking during pregnancy is prevalent. Rs-fMRI data were acquired on a 3T Skyra (Siemens, Erlangen, Germany) and pre-processed using AFNI. Pre-processing included motion correction, realignment, regression and blurring. All images were registered to the 3x3x3 mm3 Talairach-Tournoux (TT) standard space. Group independent components analysis (ICA) with 20 components and dual regression were performed in FSL. Twelve standard resting state networks (RSNs) were identified by comparison to the standard set of 20 RSNs from the Functional Connectome Project [Biswal et al., 2010]. FSL-randomise was used to identify significant clusters within each RSN [Forman et al., 1995] where connectivity between groups differed at p<0.001. We report regions that survive cluster size correction at α<0.05 using AFNI-3dClustSim with the new “mixed ACF” (autocorrelation function) methodology to account for non-Gaussianity in the spatial noise distribution [Cox et al., 2017].Results
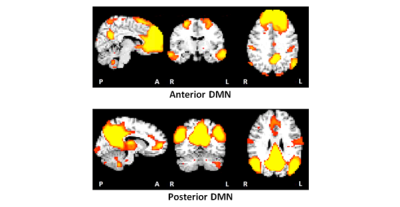
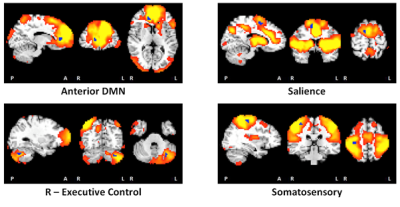
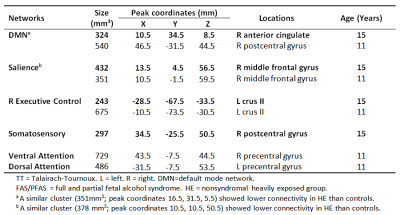
Notably, the default mode network (DMN) was split into anterior and posterior components (Figure 1). Resting state FC (RSFC) was lower in 4 GM regions within 4 RSNs in children with FAS/PFAS compared to controls (Figure 2), including the right (R) anterior cingulate within the anterior DMN, the R middle frontal gyrus within the salience network, left (L) crus II within the R executive control network and R postcentral gyrus within the somatosensory network. Lower RSFC within the DMN and salience network was also seen in adolescents with HE compared to controls in regions that overlapped with the FAS/PFAS-derived clusters. Cluster sizes and peak coordinates of each ROI showing significant group differences are given in Table 1, together with regions where differences were seen at 11 years.Discussion
Although these subjects have reached adolescence, the two distinct DMNs often seen during childhood was still evident [de Bie et al., 2012]. This could be due to delayed maturation in children with FASD and requires further investigation. FC reductions in the salience and R executive control networks seen previously at 11 years [Fan et al., 2017] were evident in the same regions in adolescence, as was RSFC reductions within DMN, albeit now in an anterior cingulate region. Although no differences were seen in the somatosensory network at 11 years, the region showing lower FC in this network (peak coordinates: 34.5, -25.5, 50.5) at this age is close to that implicated as part of the DMN (peak coordinates: 46.5, -31.5, 44.5) at 11 years. Group differences seen in the dorsal and ventral attention networks in childhood were no longer evident in adolescence.Conclusion
Prenatal alcohol-related differences within three RSFC networks (DMN, salience and R executive control) observed previously in childhood continued to be evident in adolescence and may reflect permanent damage. In contrast, the fact that deficits in two higher-order networks (dorsal and ventral attention) were no longer evident in adolescence suggests that the group differences seen at the earlier age were attributable to developmental delay.Acknowledgements
This study was funded by the National Institutes of Health/National Institute on Alcohol Abuse and Alcoholism (NIAAA) grants R01AA016781, R21AA017410, U01AA014790; South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation of South Africa; Medical Research Council of South Africa; University of Cape Town (UCT) and Wayne State University (WSU); and the Joseph Young, Sr., Fund from the State of Michigan.References
Biswal BB, Yetkin FZ, Haughton VM, Hyde JS (1995): Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34:537-541.
Biswal BB, Mennes M, Zuo X-N, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S (2010): Toward discovery science of human brain function. Proc Natl Acad Sci 107:4734-4739.
Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA (2017): FMRI clustering in AFNI: False positive rates redux. Brain Connect 7:152-171.
de Bie H, Boersma M, Adriaanse S, Veltman DJ, Wink AM, Roosendaal SD, Barkhof F, Stam CJ, Oostrom KJ, Delemarrevan de Waal HA (2012): Resting-state networks in awake five to eight-year old children. Hum Brain Mapp 33:1189-1201.
Fan J, Taylor PA, Jacobson SW, Molteno CD, Gohel S, Biswal BB, Jacobson JL, Meintjes EM (2017): Localized reductions in resting-state functional connectivity in children with prenatal alcohol exposure. Hum Brain Mapp 38:5217-5233.
Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC (1995): Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med 33:636-647.
Greicius MD, Kiviniemi V, Tervonen O, Vainionpaa V, Alahuhta S, Reiss AL, Menon V (2008): Persistent default-mode network connectivity during light sedation. Hum Brain Mapp 29:839-847.
Jacobson SW, Stanton ME, Molteno CD, Burden MJ, Fuller DS, Hoyme HE, Robinson LK, Khaole N, Jacobson JL (2008): Impaired eyeblink conditioning in children with fetal alcohol syndrome. Alcohol Clin Exp Res 32:365-372.
Supekar K, Musen M, Menon V (2009): Development of large-scale functional brain networks in children. PLoS Biol 7:1521.
Thomason ME, Dennis EL, Joshi AA, Joshi SH, Dinov ID, Chang C, Henry ML, Johnson RF, Thompson PM, Toga AW (2011): Resting-state fMRI can reliably map neural networks in children. Neuroimage 55:165-175.
Figures