0588
Short-range functional connections at birth predict neurodevelopmental outcome at 2 years of ageMinhui Ouyang1, Qinmu Peng1,2, Michelle Slinger1, and Hao Huang1,2
1Radiology, Children's Hospital of Philadelphia, Philadelphia, PA, United States, 2Radiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States
Synopsis
Short-range functional connectivity (FC) is the major contributor to overall significant FC increases around birth. With
Purpose
Information transfer in the human brain at a macroscopic level arises from both interactions between adjacent areas, referred to as “short-range” connections, and projections from distant areas, referred to as “long-range” connections [1, 2]. During early brain development from 31-42 postmenstrual weeks (PMW), increases of functional connections (FC) were primarily found in short-range FC [3], while the development of long-range FC occurs mostly after birth [4, 5]. With critical role of short-range FC in neonate brain maturation, we hypothesized that short-range FC at birth predicts the neurodevelopmental outcome at 2 years of age. In this study, we aimed to investigate the role of short-range FC across the brain regions obtained from neonatal resting state fMRI (rs-fMRI) in predicting cognitive score at 2 years of age using machine learning algorithms.Methods
Participants, acquisition of rs-fMRI at birth and neurodevelopmental assessment at 2 years of age: 26 normal preterm and term neonates (Male/Female: 19/7) were recruited and scanned around birth (ages of 36.2±2.7PMW). All MR scans were performed on a 3T Philips Achieva MR system. For rs-fMRI, 210 whole brain EPI volumes were acquired using the following parameters: TR/TE=1500/27ms, FOV=168×168 mm2, voxel size=2.4×2.4×3mm3, 30 slices. Co-registered T2-weighted images were also acquired. At their 2 years of age (corrected for prematurity), the participating infants underwent a Bayley test (Bayley-III) [6] to assess their cognitive, language and motor development. Rs-fMRI preprocessing and quantification of short-range and long-range FC: All neonate images were nonlinearly registered to a 37PMW brain template [7] using contrasts of T2-weighted images. Rs-fMRI preprocessing included removal of the first 15 time points, slice-timing, head-motion correction, co-registration, spatial normalization, spatial smoothing, detrending, temporal filtering and regression of nuisance variables. Short-range and long-range functional connectivity strength (FCS) were calculated as the average of the correlations between one voxel and its neighborhood voxels (≤ 20mm) or distant voxels (>20mm), respectively (Fig.1). Feature extraction: The template brain cerebral cortex was randomly subdivided into 256 regions with equal size [8]. Super-voxel short-range and long-range FCS values were calculated by averaging the FCS in each region for all subjects. Cognitive score prediction: Pattern analysis was performed using the support vector regression (SVR) algorithm implemented in LIBSVM (http://www.csie.ntu.edu.tw/~cjlin/libsvm) [9]. Cognitive score from Bayley-III was used as the training measure. Super-voxel short-range or long-range FCS values formed the feature vector and was used as the SVR predictor. Leave-one-out cross-validation (LOOCV) was adopted to evaluate the performance of the SVR model. In this procedure, the cognitive score of each infant was predicted from an independent training sample, without being used to build the prediction model. Pearson correlation coefficient between the actual and predicted cognitive score was computed to assess the prediction accuracy.Results
Averaged short-range and long-range FCS maps of 26 neonate brain are shown in Fig.1. Notably, primary sensorimotor regions show strong short-range FC and long-range FC is relative weak across entire cortex. With short-range FCS as features, the predicted cognitive score obtained from SVR models is significantly correlated (r=0.56, p=0.003) with the actual cognitive score from Barley-III, shown in Fig 2a. In the SVR model, the training data is defined as a weighted combination of all features and the weight vector indicates the relative contribution of each feature. A heterogeneous feature weight, i.e. feature contribution pattern, across cerebral cortex can be appreciated in Fig 2b, with red-color cortical areas more important in prediction. However, with long-range FC as features, significant prediction was not achieved (r=0.06, p=0.74). Fig 3a shows the top ten cortical regions that contribute most among the features with highest 20% feature weights in the SVR model. The scatter plot in Fig 3b shows the short-range FCS from a representative region, cuneus gyrus, is significantly correlated (r=0.56, p=0.004) with the actual cognitive score at 2 years of age.Discussion and conclusion
We found that short-range FC at birth predicts neurodevelopmental outcome at 2 years of age. The prediction feature weight is heterogeneous across the cortical regions (Fig 2b) with its distribution pattern distinguished from FCS distribution patterns (Fig 1). The prediction feature weight map indicates differentiated roles among cortical regions in future cognitive maturation. The short-range FCS at cortical regions with highest prediction feature weight may be used as the predictive biomarkers for cognitive score at 2 years of age. With short-range FCS quantifying functional maturation, significant correlation between short-range FCS and cognitive score (e.g. Fig 3b) suggests that higher maturational level of local functional connectivity at birth predicts higher cognitive scores at 2 years of age. Analysis of language and motor scores prediction with short-range FCS is under way.Acknowledgements
This study is funded by NIH MH092535, MH092535-S1 and HD086984.References
[1] Bullmore and Sporns (2009) Nat Rev Neurosci. 9:110. [2] Ouyang et al (2017) Neurosci Biobehav Rev (in press). [3] Cao et al (2017) Cereb Cortex 27: 1949. [4] Gao et al (2011) Plos One. 6:e25278. [5] Fair et al (2009) PLoS Comput Biol. 5:e1000381. [6] Beyley (2006) San Antonio TX: Harcourt Assessment. [7] Serag et al (2012) Neuroimage 59:2255. [8] Zalesky et al (2010). Neuroimage 50: 970. [9] Chang and Lin (2011) ACM TIST 2:27Figures
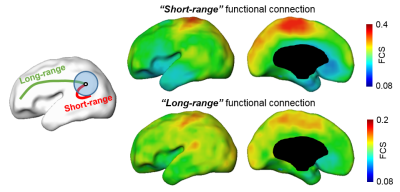
Fig 1: Left panel: short-range FC (red color) is the FC inside the neighborhood brain regions (blue circle), long-range FC is the FC (green color) outside the neighborhood brain regions. Right panel: Averaged short-range (top) and long-range (bottom) functional connection (FC) from 26 neonate brains.
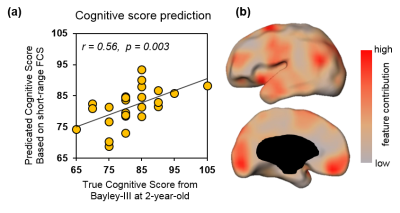
Fig 2: Prediction of cognitive score with short-range FCS as features. (a) Significant correlation (p=0.003) between predicted and true cognitive scores. (b) Visualization of feature contributions across cortical regions.
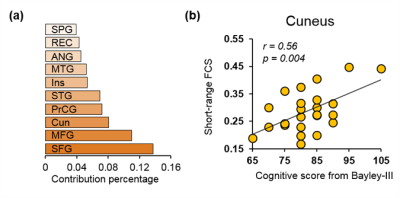
Fig 3: (a) Cortical regions that contribute most among the features with highest 20% feature weights in the SVR model. Abbreviations: SFG: superior frontal gyrus. MFG: middle frontal gyrus. Cun: Cuneus gyrus. PrCG: precentral gyrus. STG: superior temporal gyrus. Ins: insular cortex. MTG: middle temporal gyrus. ANG: angular gyrus. REC: rectus gyrus. SPG: superior parietal gyrus. (b) Significant correlation (p=0.004) between cognitive score and short-range FCS in cuneus gyrus, a representative cortical region listed in (a).