0585
Whole Brain FLAIR Imaging at 7T Employing Universal Pulses1German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany, 2NeuroSpin, CEA, Saclay, France, 3Department of Physics and Astronomy, University of Bonn, Bonn, Germany
Synopsis
In this work, we present a fluid suppressed Turbo-Spin-Echo sequence for ultra-high-field application. To obtain images comparable in contrast and homogeneity with 3T results, we replaced all RF pulses with universal parallel transmit kT-point pulses. During the imaging session, no pulse calculations or complex B1 shimming procedures are necessary, making this approach a promising tool for clinical application.
Introduction
In the last years, UHF (7T) imaging has become an increasingly important modality for neuroscience applications. However, the lack of a fast T2 weighted FLAIR imaging protocol for lesion detection often requires subject-rescan at lower field (3T), as various issues compromise image quality and scanning efficiency at UHF: Prolonged tissue T1 relaxation times lead to a loss of T2 weighting, B1/B0 inhomogeneities induce signal drop outs and/or insufficient suppression of unwanted tissue components, and SAR limitations reduce scanning efficiency. To overcome the aforementioned limitations we propose a new FLAIR imaging technique employing universal parallel transmit kT-point pulses1 .Methods
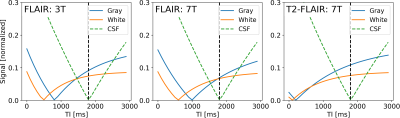
Imaging experiments were performed on a MAGNETOM 7T scanner with parallel transmit capabilities (Siemens AG, Healthcare Sector, Erlangen, Germany) using a head array coil with 32 receive and 8 transmit channels (Nova Medical, Wilmington, USA). Imaging was performed using an UHF optimized variable flip angle (vFA) TSE2, preceded by a T2-prep module3 for fluid suppression (see Fig. 1).The implemented universal parallel transmit kT-point pulses were optimized to produce uniform excitation and refocusing over the whole brain4. The optimization was based on data derived from a population of adults in a pre-study, rendering RF calibration procedures unnecessary during the actual imaging session.The variable flip angle trains were optimized to yield highest possible SNR for a predefined signal shape (based on extended phase graph calculations). The target signal shape was chosen to reduce T1 weighting in the echo train and introduce T2 contrast. To further enhance T2 weighting a T2-prep module was implemented instead of a standard adiabatic inversion pulse (see Fig. 2).To keep scan time as short as possible, an optimized linear reordering scheme with elliptical scanning was used5. It allows to choose the turbofactor (number of refocusing pulses per TR) independent of the phase and partition encoding. Imaging was performed in one healthy volunteer. FLAIR sequence parameters: TR = 7000 ms, TI/TE = 1180 / 100 ms turbofactor 320, 1x1x1 mm resolution, matrix size 256x256x176 (whole brain coverage), GRAPPA (R=2), total scan time ≈ 6 min.
Results
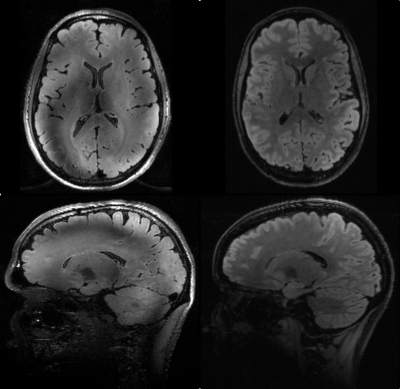
Figure 3 shows FLAIR images acquired with and without UHF optimizations. On the left a FLAIR image using a standard single channel protocol employing adiabatic inversion is depicted. The image clearly lacks T2 contrast. Furthermore, artifacts due to insufficient inversion are present. On the right, the universal T2-prep module creates T2 weighting, resulting in images with similar contrast as the “gold standard” at 3T. Additionally, due to the B1 insensitivity of the universal pulses, no artifacts originating from incomplete inversion/refocusing are visible. As can be seen in Fig. 3 (bottom right) even down to the cerebellum effective inversion can be maintained and the image appears significantly more homogeneous over the brain. However, the applied inversion time of 1180 ms is far below the calculated value indicating that the refocusing angle in the T2-prep module is smaller than 180 degree during the magnetization preparation. This phenomenon is currently under investigation.Discussion/Conclusion
It is shown that universal pulses enhance image quality in 3D whole brain dark-fluid imaging at 7T. In combination with the universal T2-prep module the proposed method allows for the acquisition of homogeneous FLAIR images with T2 weighted contrast similar to 3T. In contrast to standard parallel transmit imaging protocols, no adjustments (such as B1 shimming) or pulse calculations are needed during the imaging session, keeping the workflow lightweight and straightforward.Acknowledgements
No acknowledgement found.References
1. Gras et al. Magn Reson Med. 2017;77,635-642
2. Pracht et al. Magn Reson Med. 2017, doi:10.1002/mrm.26913
3. Nezafat et al. Magn Reson Med. 2009;61,1326-1335
4. Gras et al. PLoS One. 2017;12, doi:10/1371/journal.pone.0183562
5. Feiweier T. US Patent 7,728,588 B2
Figures