0582
Contrast-enhanced MR microscopy of amyloid plaques in five mouse models of Alzheimer’s disease: comparison with amyloid plaques detection in human brains.1Molecular Imaging Research Center (MIRCen), Commissariat à l'énergie atomique et aux énergies alternatives (CEA), Fontenay aux Roses, France, 2INSERM U986, Université Paris-Sud, Fontenay-aux-Roses, France, 3Institut des Maladies Emergentes et des Therapies Innovantes (IMETI), Commissariat à l'énergie atomique et aux énergies alternatives (CEA), Fontenay aux Roses, France
Synopsis
Gadolinium(Gd)-stained MRI is based on Gd-contrast agent administration into the brain. This method significantly improves the detection of amyloid plaques, one of the lesions of Alzheimer's disease and a potential biomarker for its diagnosis. Here, we aimed to better understand the origin of contrast induced by amyloid plaques by determining critical parameters required for their detection using five mouse models of amyloidosis presenting with different plaque typologies. Then, we showed for the first time that Gd-stained MRI can detect amyloid plaques in postmortem human brain tissues and compared the detection achieved in mice with those obtained in human samples.
Introduction
Amyloid plaques are one of the early lesions associated to Alzheimer's disease (AD). Detection of these lesions by neuroimaging is a very promising biomarker approach for AD diagnosis and evaluation of the efficacy of potential therapies. Therefore, numerous efforts aim to develop non-invasive methods, such as high-resolution magnetic resonance imaging (MRI), for amyloid plaques imaging. Gd-stained MRI allows to label these lesions in vivo1-4 or ex vivo4,5 in mouse models of amyloidosis or in primates6. This method is based on the intracerebral injection of a clinically approved MRI contrast agent (Dotarem®), which rapidly diffuses throughout the brain parenchyma and increases the signal and contrast of MR images. Numerous transgenic mouse strains have been developed to mimic a range of AD-related pathologies including different types of amyloid lesions7. These models have been a key feature in translational research, providing significant insights into the pathophysiology of AD. However, none of these models replicates the full spectrum of AD and comparison of plaques from animal models and humans detected by various imaging methods is a recurrent question. In this study, we investigated the extent to which Gd-stained MRI allows the detection of different types of amyloid lesions including focal, diffuse, intracellular and vascular amyloid deposits. For that, we used five mouse models of amyloidosis (APPSL/PS1M146L, APP/PS1dE9, APP23, APPSwDI, 3xTg) presenting with different plaque typologies. After MR experiments, brains were processed by histology to assess the impact of size, compactness, and iron load of amyloid plaques on their detection by Gd-stained MRI. Then, we explored the capacity of Gd-stained MRI to detect amyloid plaques in postmortem human-AD brains and compared detection profiles determined in mice to that obtained in human-AD brains.Methods
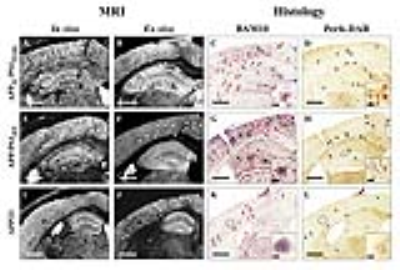
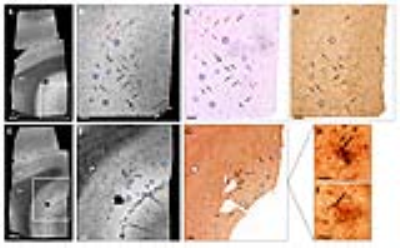
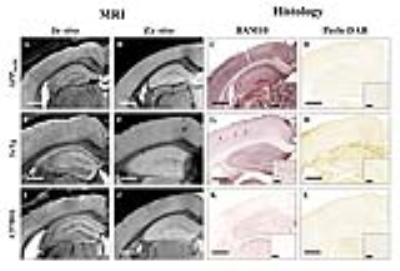
We selected five transgenic strains presenting with compact amyloid plaques (APPSL/PS1M146L (n = 6), APP/PS1dE9 (n = 6) and APP23 (n = 4)), diffuse amyloid plaques (APPSwDI (n = 6)) and intracellular amyloid deposits (3xTg (n = 7)). C57Bl/6 amyloid-free mice (n = 2) were used as controls. In vivo and ex vivo Gd-stained MR images were recorded in each mouse model with a 7T spectrometer at a resolution of 29×29×117µm3 in vivo and 25×25×100µm3 ex vivo. Gd-stained MR images of human brain samples from the cerebral cortex and adjacent white matter of three AD patients and one control were also recorded postmortem. Then, all brain samples were evaluated by histology using an anti-Aβ staining (BAM10 antibody and Congo Red) and an iron staining (Perls-DAB coloration). Registration between MR images and histological sections allowed to study critical parameters associated with plaques detection: size, compactness and iron load of plaques.
Results
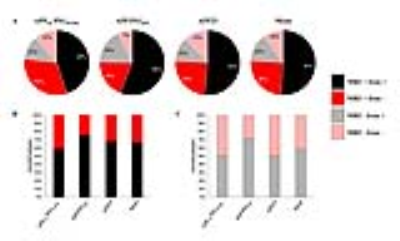
Histological evaluation revealed that morphology of amyloid plaques is similar in APPSL/PS1M146L, APP/PS1dE9, APP23 and human-AD brains studied (Fig. 1, 2) while they strikingly differ in other mouse models (Fig. 3). In these models, we determined that 76% of cortical amyloid plaques seen on histological sections were detected by Gd-stained MRI (Fig. 4).Registration between MR images and histological sections showed that only compact amyloid plaques were detected by in vivo or ex vivo Gd-stained MRI (Fig. 1, 2). Diffuse plaques, intracellular amyloid deposits and amyloid angiopathy were never detected (Fig. 3). The minimal plaque sizes resolvable by Gd-stained MRI were 36µm in vivo and 30µm ex vivo in mice and 25µm in human-AD brain samples. Finally, our study showed that iron accumulation is not necessary for plaque detection after Gd-staining. Indeed, in mice, 33% of the amyloid plaques detected by Gd-stained MRI contained little or no iron (Fig. 4). Also, in human-AD brain samples, although most of the human plaques contained iron deposits some plaques devoid of iron were detected. Whereas, in most in vivo studies without contrast agent, iron is considered as a critical parameter for plaque detection8,9.Discussion and conclusion
This study clearly highlights differences among amyloid plaques found in different mouse models of amyloidosis, and provides a better understanding of the origin of contrast induced by amyloid plaques by Gd-stained MRI. We demonstrated that the ability to detect amyloid plaques by Gd-stained MRI is strikingly different in the various mice models of amyloidosis studied or in humans. We also showed that Gd-stained MRI can be used to detect amyloid lesions in models with large compact amyloid plaques such as APPSL/PS1M146L, APP/PS1dE9 or APP23 mice independently of their iron load, but not in models with large diffuse Aβ deposits (i.e. APPSwDI) or small intracellular amyloid aggregates (i.e. 3xTg mice). Here, we suggest that detection of amyloid plaques by Gd-stained MRI in APPSL/PS1M146L and APP/PS1dE9 is the most similar to that in human-AD brains. Finally, we showed for the first time that Gd-stained MRI can detect amyloid plaques in postmortem human brains tissues.
Acknowledgements
No acknowledgement found.References
1. Petiet A. et al. Gadolinium-staining reveals amyloid plaques in the brain of Alzheimer’s transgenic mice. Neurobiol Aging. 2012 Aug;33(8):1533-44.
2. Santin M. D., Debeir T., Bridal S. L., Rooney T., Dhenain M. Fast in vivo imaging of amyloid plaques using μ-MRI Gd-staining combined with ultrasound-induced blood-brain barrier opening. Neuroimage. 2013 Oct 1;79:288-94.
3. Santin M. D. et al. In vivo detection of amyloid plaques by Gadolinium-stained MRI can be used to demonstrate the efficacy of an anti-amyloid immunotherapy. Front Aging Neurosci. 2016 Mar 22;8:55.
4. Dudeffant C., et al. Contrast-enhanced MR microscopy of amyloid plaques in five mouse models of amyloidosis and in human Alzheimer's disease brains. Sci Rep. 2017 Jul 10;7(1):4955.
5. Dhenain M., Delatour B., Walczak C., Volk A. Passive staining: a novel ex vivo MRI protocol to detect amyloid deposits in mouse models of Alzheimer’s disease. Magn Reson Med. 2006 Mar;55(3):687-93
6. Bertrand A. et al. Micro-MRI study of cerebral aging: ex vivo detection of hippocampal subfield reorganization, microhemorrhages and amyloid plaques in mouse lemur primates. PLoS One. 2013;8(2):e56593.
7. Duyckaerts C., Potier M.C., Delatour B. Alzheimer disease models and human neuropathology: similarities and differences. Acta Neuropathol. 2008 Jan;115(1):5-38.
8. Jack C.R. Jr. et al., In vivo visualization of Alzheimer's amyloid plaques by magnetic resonance imaging in transgenic mice without a contrast agent. Magn Reson Med. 2004 Dec;52(6):1263-71.
9. Dhenain M. et al., Characterization of in vivo MRI detectable thalamic amyloid plaques from APP/PS1 mice. Neurobiol Aging. 2009 Jan;30(1):41-53.
Figures