0579
7T MRI allows detection of disturbed cortical layers in medial temporal lobe in patients with Alzheimer’s disease1Radiology, Leiden University Medical Center, Leiden, Netherlands, 2Human Genetics, Leiden University Medical Center, Leiden, Netherlands, 3Anatomy and Neurosciences, Amsterdam Neuroscience, VU University Medical Center, Amsterdam, Netherlands, 4Percuros BV, Leiden, Netherlands
Synopsis
Using 7T T2*-w imaging, we scanned post-mortem hemispheres of aged controls and patients with Alzheimer’s disease (AD) to assess the potential of MRI for anatomical cortical parcellation of the medial temporal lobe based on
Introduction
The human cerebral cortex is constructed of distinct cyto-architectural layers with myelinated layers running tangentially to the cortical surface. The myelo- and cytoarchitecture differ per area, but are consistent among individuals, which makes parcellation of distinct functional areas possible.1,2
Advances in 7T MRI have made it possible to image micro-anatomic structures including some hippocampus substructures and the layered structure of the neocortex with highly myelinated bands.3-5 However, the resolution of these in vivo scans did not allow delineation of anatomically distinct areas. In this study we scanned post-mortem whole brain hemispheres with a high-resolution T2*-w protocol, to assess the potential of MRI for anatomical parcellation based on myelo- and cytoarchitectural contrast.
Cortical lamination is not only of interest in the context of structural and functional neuroanatomy. In patients with Alzheimer’s disease, distortion of the cortical lamination has been observed histologically.6 Presently, there is no literature available on the visibility of these laminar alterations on MRI and the regional variation of cortical lamination in AD. Additionally, assessment of lamination changes may provide new information on involved neuroanatomical layers and their relation to the pathogenesis of AD.
Methods
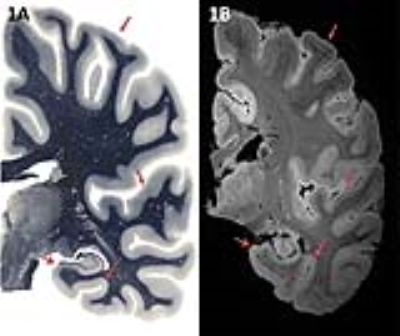
Unilateral hemispheres of 15 healthy controls and 16 AD patients were imaged on a Philips Achieva 7 Tesla MRI system. T2*-w images with a 0.3mm isotropic resolution were acquired using a 3D Gradient Echo sequence (TR = 36 ms, TE = 20 ms, Flip angle = 10°). The medial temporal lobe, including the hippocampus was segmented by a neuro-anatomist (WvdB) based on visible anatomical contrast on MRI, as described in results, with comparison to the Paxinos atlas7 (Fig. 1). Subsequently, segmented images of AD patients were assessed for alterations in the cyto- and myeloarchitecture compared to age-matched controls. Additionally, histological stainings for cytoarchitecture (Nissl) and myelination (PLP) were performed on tissue of one control and one AD-hemisphere.Results
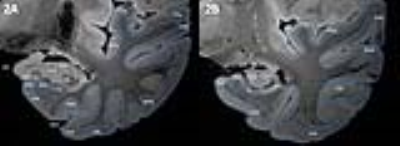
Ultra-high field 7T MRI provides the spatial resolution and contrast that is needed to detect cortical layers in the medial temporal lobe (MTL; Fig 1) and makes segmentation of MTL subregions possible (Fig 2). The CA1-4 layers of the hippocampus can be segmented based on shape, cortical thickness and hyperintensity in the ammon’s horn and transition to subiculum was clearly visible. The subiculum, presubiculum (PrS) and parasubiculum can be distinguished and reveal three layers on the scan. Cortical lamination is also visible in the parahippocampal gyrus (PHG) and fusiform gyrus (FuG), in particular layers 1-4 can be distinguished. Furthermore, the layers of the superior temporal gyrus (STG) and anterior and posterior transverse temporal gyrus (TTG1 and TTG2) are visible.
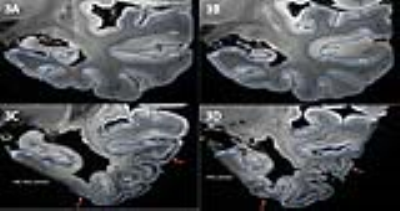
Segmentation and parcellation was also feasible in AD scans (Fig. 3). In scans of AD patients, atrophy of the MTL subregions is apparent; in particular of the hippocampus, subiculum, entorhinal cortex, PHG and MTG. Noteworthy, clear contrast between the layers in the hippocampus and neocortical subregions was seen in mild and advanced AD. Hypointensity of the deeper layers of part of the PHG, FuG and middle temporal gyrus (MTG) was observed in advanced AD (Fig 3, arrows), whereas TTG1 and TGG2 appeared more spared.
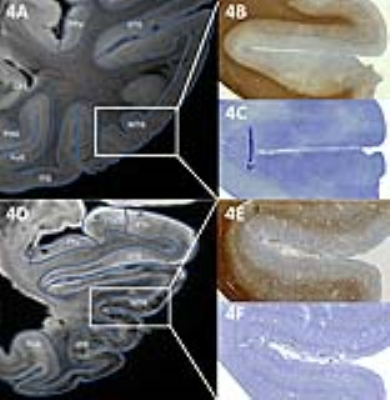
Fig. 4 shows high correspondence between the cortical contrast on MRI and myelination in the same area. The abnormal hypointense band visible in the MTG of the AD hemisphere (Fig 4D) corresponds to a thick myelinated band in the cortex on the PLP staining (Fig 4E). This band is not necessarily confined to cortical areas that are already heavily myelinated; e.g. the FuG, which is sparsely myelinated, showed a dense hypo-intense mid-cortical layer in severe AD cases (Fig. 3C-D).
Discussion and conclusion
As a result of increased scanning time and less motion induced artefacts, 7T T2*-w MRI of post mortem hemispheres has provided sufficient spatial resolution and contrast to segment MTL subregions of both controls and AD patients, based on cortical layering and thickness. Changes in intensity in subregions of the MTL and distortion of cortical lamination are observed in AD patients, and correspond with myelin. Previous work showed that in the cortex myelination highly correlated to the presence of iron8, which explains sensitivity of T2*-w scans to cortical lamination. Therefore, MRI can provide information on cortical lamination in AD and translation of the results to in vivo protocols may lead to additional biomarkers in the study of ADAcknowledgements
This work was financially supported by a grant from the European Union 7th Framework Program: BrainPath (PIAPP-GA-2013–612360).
Netherlands Brain Bank (Amsterdam, The Netherlands) and Normal Aging Brain Collection (NABC) for supplying brain tissue.
Netherlands Organisation for Scientific Research (NWO), VIDI research program, project ‘’Amyloid and vessels’’, number 864.13.014.
References
- Brodmann, K., Vergleichende Lokalisationslehre Der Großhirnrinde. J.A. Barth, Leipzig. 1909.
- Vogt, O., Zur anatomischen Gliederung des Cortex cerebri. J. Psychol. Neurol. 1903;160–180.
- Barazany, D., Assaf, Y., 2012. Visualization of cortical lamination patterns with magnetic resonance imaging. Cereb. Cortex 22, 2016–2023. doi:10.1093/cercor/bhr277
- Fracasso, A., van Veluw, S.J., Visser, F., Luijten, P.R., Spliet, W., Zwanenburg, J.J.M., Dumoulin, S.O., Petridou, N., Lines of Baillarger in vivo and ex vivo: Myelin contrast across lamina at 7T MRI and histology. Neuroimage. 2016;133, 163–175. doi:10.1016/j.neuroimage.2016.02.072
- Kerchner, G.A., Hess, C.P., Hammond-Rosenbluth, K.E., Xu, D., Rabinovici, G.D., Kelley, D.A.C., Vigneron, D.B., Nelson, S.J., Miller, B.L., Hippocampal CA1 apical neuropil atrophy in mild Alzheimer disease visualized with 7-T MRI. Neurology. 2010;75, 1381–1387. doi:10.1212/WNL.0b013e3181f736a1
- Brun, A., Englund, E., Regional pattern of degeneration in Alzheimer’s disease: neuronal loss and histopathological grading. Histopathology 1981;5, 549–564. doi:10.1111/j.1365-2559.1981.tb01818.x
- Mai, J.K., Majtanik, M., Paxinos, G., n.d. Atlas of the human brain
- Bulk M, Abdelmoula WM, Nabuurs RJA, Van Der Graaf LM, Mulders CWH, Mulder AA, et al. Post mortem MRI and histology demonstrate differential iron accumulation and cortical myelin organization in early and late onset Alzheimer’s disease. Neurobiol Aging . 2017. In press, Accepted Manuscript
Figures