0565
All-In-One MRI of Atherosclerosis: Towards Higher Clinical Value1Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 2Department of Bioengineering, University of California, Los Angeles, Los Angeles, CA, United States, 3Department of Medicine, University of California, Los Angeles, Los Angeles, CA, United States, 4Department of Radiology, Anzhen Hospital, Capital Medical University, Beijing, China
Synopsis
Multi-contrast MRI is a promising yet under-utilized imaging modality for evaluating the disease status of atherosclerosis. Major drawbacks of conventional protocols include long complex scan procedures and variability in image interpretation due to the qualitative nature of the images. In this
Introduction
Excellent soft tissue visualization and versatile contrast mechanisms allow MRI to provide rich diagnostic information regarding the disease status of atherosclerosis, including lumen stenosis, plaque morphology and composition. However, multi-contrast MRI of atherosclerosis has yet to fully realize its early promise and remains under-utilized clinically. Major roadblocks include complex, costly scanning procedures and difficulty in image interpretation due to its qualitative nature. Quantitative methods such as T1 and T2 mapping can offer high reproducibility and portability of the results, but typically require even longer scan time.1,2 Recently, we proposed the concept of Quantitative Multi-contrast Atherosclerosis Characterization (qMATCH),3 in order to achieve comprehensive and quantitative assessment of atherosclerosis including MRA, dark-blood vessel wall morphology, and T1/T2 mapping, all in one 8-minute scan. In this paper, we report the preliminary in vivo results of qMATCH in healthy subjects and patients with known carotid atherosclerosis.Methods
The proposed qMATCH technique was formulated based on the low-rank tensor (LRT) framework,4 exploiting the partial separability of spatial and contrast dimensions in the multi-contrast images to achieve vast acceleration. The pulse sequence consisted of T2-IR preparation modules with various durations and a continuous spoiled gradient echo train to generate a range of different T2 and T1 weightings, respectively. A Gaussian random variable-density sampling scheme was designed for capturing the contrast dynamics. Additional details of the sequence design and reconstruction process were summarized in a recent publication.3
All imaging data were acquired with a clinical 3T scanner (MAGNETOM Verio, Siemens Healthineers) using a 4-channel phased-array dedicated carotid coil. Imaging parameters include: a coronal 3D slab covering both carotid arteries, FOV=150x150x26mm3, spatial resolution=0.7mm isotropic, flip angle=8°, bandwidth=343Hz/pixel, TEs=20/30/40/50/60/70ms, scan time=8mins. Initial validation was performed using a custom built relaxometry phantom made with agarose and nickel chloride at various concentrations. 5 Standard spin echo sequence was used as the reference for relaxometry in phantom. In vivo imaging was performed in 14 normal subjects and 9 patients with known carotid atherosclerosis. MOLLI 5 and T2-SSFP 6 were used in the normal subjects as the reference method for in vivo T1 and T2 relaxometry, respectively. Three patients further underwent clinically indicated carotid endarterectomy and their plaque specimens were harvested during the surgery. Histological study of the specimen was performed as the diagnostic reference for qMATCH.
Results
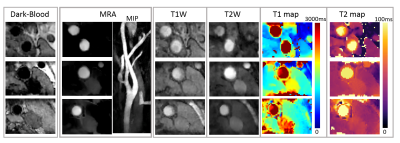
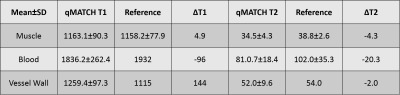
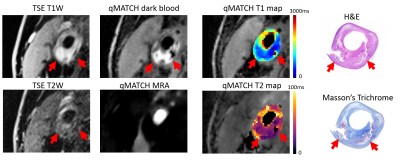
In the phantom study, T1 and T2 quantification by qMATCH showed high correlation with the spin echo reference (Pearson's r=0.97, p<0.01). The mean bias by qMATCH was -3.4% in T1, and -8.2% in T2, respectively. In the in vivo study, qMATCH was able to generate multi-contrast images including bright-blood MRA, dark-blood wall morphology, T1/T2-weighted images and mapping, as shown in a representative dataset in Figure 1. T1/T2 quantification by qMATCH in the normal subjects was compared with reference methods (MOLLI and T2-SSFP) and literature values 1,7 in three tissue types, showing excellent consistency (Figure 2). A representative qMATCH dataset with possible intra-plaque hemorrhage is shown in Figure 3, in comparison with conventional multi-contrast MRI images and histological reference.
Discussion
Preliminary results from the in vivo studies demonstrated the feasibility of “all-in-one” assessment of carotid atherosclerosis using qMATCH. Multiple lesion characteristics can be assessed simultaneously, including luminal stenosis (by bright-blood MRA), plaque burden (by dark-blood images), and plaque composition (by T1/T2 weighted images and mapping). Compared with conventional multi-contrast plaque imaging protocols, qMATCH also had the advantages of 3D isotropic resolution, large anatomical coverage, and inherently co-registered multi-contrast image set. It not only reduced scan time, but also allowed quantitative relaxometry mapping which may potentially increase the diagnostic accuracy, both of which would lead to improved value of MRI. Future work will focus on evaluating the diagnostic performance and reproducibility of qMATCH in a larger cohort with histological reference.Conclusion
Quantitative multi-contrast MRI for comprehensive atherosclerosis evaluation in a single scan is feasible with qMATCH.Acknowledgements
This research project was supported by National Heart, Lung, and Blood Institute (Grant No.: R01 HL096119).References
1. Biasiolli, L., Lindsay, A. C., Chai, J. T., Choudhury, R. P. & Robson, M. D. In-vivo quantitative T2 mapping of carotid arteries in atherosclerotic patients: segmentation and T2 measurement of plaque components. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance 15, 69, doi:10.1186/1532-429X-15-69 (2013).
2. Coolen, B. F. et al. Three-dimensional quantitative T1 and T2 mapping of the carotid artery: Sequence design and in vivo feasibility. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 75, 1008-1017, doi:10.1002/mrm.25634 (2016).
3. Xie, Y. et al. Quantitative multi-contrast atherosclerosis characterization: comprehensive quantitative evaluation of atherosclerosis in a single scan. In Proceedings of the 25th Annual Meeting of ISMRM, Honolulu, Hawai'i, USA (2017);
4. Christodoulou, A. et al. A general low-rank tensor framework for high-dimensional cardiac imaging: application to time-resolved T1 mapping. In Proceedings of the 24th Annual Meeting of ISMRM, Singapore (2016);
5. Messroghli, D. R. et al. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 52, 141-146, doi:10.1002/mrm.20110 (2004).
6. Giri, S. et al. T2 quantification for improved detection of myocardial edema. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance 11, 56, doi:10.1186/1532-429X-11-56 (2009).
7. Wang, J. et al. Simultaneous noncontrast angiography and intraplaque hemorrhage (SNAP) imaging for carotid atherosclerotic disease evaluation. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 69, 337-345, doi:10.1002/mrm.24254 (2013).
Figures