0562
Characterizing the sensitivity of ihMT for various dipolar relaxation times (T1D) at high RF power using frequency-alternated and cosine-modulated RF pulses for dual frequency-offset saturation1Aix-Marseille Univ, CNRS, CRMBM, Marseille, France, 2APHM, Hôpital Universitaire Timone, CEMEREM, Marseille, France, 3Radiology, Division of MR Research, Beth Israel Deaconess Medical Center, Harvard Medical Schooll, Boston, MA, United States
Synopsis
In the present study, we used both numerical simulations and experiments performed in a preclinical setup to characterize the mechanisms of the ihMT boost effect (signal enhancement achieved using concentrated bursts of high-power RF for saturation). We demonstrated that the ihMT boost effect depends on T1D and is more intense in short-T1D components. This feature allowed, using various strategies for dual frequency-offset RF saturation, different ihMT contrasts to be produced and revealed strong ihMT signal outside the brain.
Purpose
Inhomogeneous Magnetization Transfer (ihMT)1 is a T1D-weigthed imaging technique2 with T1D, the relaxation time of the dipolar order. The latter corresponds to the polarization of dipolar coupled spins within their local magnetic fields.3,4 In an ihMT experiment (Fig.1a), sensitization to T1D is achieved by subtraction of a MT image (MTsingle) obtained with a single frequency-offset RF saturation (+Δf, RF pulse-power, b1), which, in the limit where γ.b1.T1D>0.01, contains dipolar order effects5, and a MT image (MTdual) obtained with a symmetric dual frequency-offset RF saturation (±Δf, RF-pulse power, b1), which ideally eliminates dipolar order contribution.2 Long T1D values estimated in lipid bilayer membranes6,7 most likely explain the pronounced specificity of ihMT images for myelin (Fig.1b).8–10 A recent clinical study demonstrated that a significant ihMT signal enhancement (namely “ihMT boost effect”) can be achieved using concentrated bursts of high-power RF pulses for saturation separated by relaxation periods (Fig.1c).11 In the present study, we used both numerical simulations and experiments performed in a preclinical setup to characterize the mechanisms of the ihMT boost effect at high RF power. We demonstrated that the ihMT boost effect depends on T1D and is more intense in short-T1D components. This feature allowed, using various strategies for dual frequency-offset RF saturation, different ihMT contrasts to be produced and revealed strong ihMT signal outside the brain.Methods
An ihMT sequence consisting in a saturation preparation using bursts of concentrated RF power followed by a single-shot RARE readout (TR/TE=3.4s/1.82ms, mtx: 64x64, FOV=2x2mm, single slice, thickness 1mm) (Fig.1c) was computed on a 11.75T preclinical system (Bruker Avance 500) and applied on 3 mice. All experiments were performed with frequency offsets Δf=±10kHz; and a saturation time τ=0.9s. Dual frequency-offset saturation was achieved by using either frequency-alternating RF pulses (PW/Δt=1/1.3ms), which filtered components with T1D<1.3ms from the ihMT signal, or by cosine-modulated pulses (PW=1ms), which led to simultaneous saturation at both offsets and no T1D-filtering.10 The ihMT boost effect was characterized by varying the burst repetition time (BTR) for different values of the number of pulses within each burst (Np). For each investigated {Np, BTR} combination, the average power of individual RF pulses (b1) was adjusted to produce a fixed B1,RMS=6.7μT over τ. Numerical simulations of ihMTR corresponding to the various configurations investigated experimentally were performed using Matlab (The MathWorks, Natick, MA) by numerical integration of the two-pool model including a dipolar reservoir and with estimated quantitative MT parameters corresponding to internal capsule and muscle.2Results and Discussion
The ihMT boost effect (rising and falling curve of ihMTR with BTR) is a general feature obtained regardless of T1D values and the type of dual frequency-offset saturation (Fig. 2). The observed experimental variations of ihMTR with {Np, BTR} were expected from theory as indicated by the generally good agreement between acquired data and simulated curves (Fig. 3). Lower simulated absolute ihMTR values could arise from poorly estimated quantitative MT parameters. The ihMT boost effect shows strong dependence with T1D. It is more intense for short-T1D components as evidenced by the relative signal gain obtained for muscle (~3 for frequency-alternated pulses, Fig. 2a; ihMTR varying from ~2.3% up to 7%) compared to internal capsule (~1.8, ihMTR varying from ~6% up to 10.5%). Dual-offset saturation performed with cosine-modulated pulses led to stronger boost effect but also to loss of contrast between different T1D components (ihMTRs reach values up to 30% in both internal capsule and muscle, Fig.2b). Finally, for the most concentrated RF power configuration, the two variants of dual frequency-offset saturation led experimentally and theoretically to fundamental different behaviors with respect to changing Np: whereas Np=2 frequency-alternated pulses led to a loss of the boost effect (Figs. 2a,3a), use of Np=1 cosine-modulated pulse maximized ihMT (Figs. 2b,3b). This is explained by the inefficiency of high b1 frequency-alternated RF pulses to produce a genuine dual saturation, free of dipolar order contribution. Strong dipolar order effects created by individual pulses (γ.b1.T1D>>0.01) could not be compensated by the frequency alternation, making the MTdual signal tending towards the MTsingle signal (Fig.4a). Reduced values of b1 used with Np=12 frequency-alternating pulses allowed recovering efficiency for frequency-alternated dual saturation (MTdual<<MTsingle) (Fig.4b). Conversely, due to simultaneous saturation at ±Δf, the efficiency of the dual frequency-offset saturation achieved by cosine-modulated pulses remained independent of b1.Conclusion
Concentration of RF power allows revealing ihMT signal in short-T1D components and leads to contrast reduction. At high RF power, frequency-alternated and cosine-modulated RF pulses are not equivalent with different T1D sensitivities. These features are important to consider to produce different contrasts in ihMT images and/or strong ihMT signal in short-T1D non-brain tissue (Fig.5).Acknowledgements
- Support from A*MIDEX grant (n°ANR-11-IDEX-0001-02) funded by the French Government “Investissements d’Avenir” program
- Support from ARSEP 2015, 2017
- Support from ANR grant (n°ANR-17-CE18-0030)
- This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No713750. Also, it has been carried out with the financial support of the Regional Council of Provence-Alpes-Côte d’Azur and with the financial support of the A*MIDEX (n° ANR- 11- IDEX-0001-02), funded by the Investissements d'Avenir project funded by the French Government, managed by the French National Research Agency (ANR)
References
1. Varma G, Duhamel G, de Bazelaire C, et al. Magnetization transfer from inhomogeneously broadened lines: A potential marker for myelin: Magn Reson Med 2015;73:614–22.
2. Varma G, Girard OM, Prevost VH, et al. Interpretation of magnetization transfer from inhomogeneously broadened lines (ihMT) in tissues as a dipolar order effect within motion restricted molecules. J Magn Reson 2015;260:67–76.
3. Provotorov B. Magnetic resonance saturation in crystals. Zh Exsp Teor Fiz 1962;14:1126–31.
4. Korb J-P, Maruani J. Pulse saturation behavior of inhomogeneously broadened lines. I. Slow spectral diffusion case. Phys Rev B 1981;23:971.
5. Manning AP, Chang KL, MacKay AL, et al. The physical mechanism of “inhomogeneous” magnetization transfer MRI. J Magn Reson San Diego Calif 1997 2016;274:125–36.
6. Swanson SD, Malyarenko DI, Fabiilli ML, et al. Molecular, dynamic, and structural origin of inhomogeneous magnetization transfer in lipid membranes. Magn Reson Med 2017;77:1318–28.
7. Varma G, Girard OM, Prevost VH, et al. In vivo measurement of a new source of contrast, the dipolar relaxation time, T 1 D , using a modified inhomogeneous magnetization transfer (ihMT) sequence: In Vivo Measurement of T 1D Using ihMT. Magn Reson Med https://doi.org/10.1002/mrm.26523.
8. Girard OM, Prevost VH, Varma G, et al. Magnetization transfer from inhomogeneously broadened lines (ihMT): Experimental optimization of saturation parameters for human brain imaging at 1.5 Tesla. Magn Reson Med 2015;73:2111–21.
9. Girard OM, Callot V, Prevost VH, et al. Magnetization transfer from inhomogeneously broadened lines (ihMT): Improved imaging strategy for spinal cord applications: ihMT for Spinal Cord Applications. Magn Reson Med 2017;77:581–91.
10. Prevost VH, Girard OM, Mchinda S, et al. Optimization of inhomogeneous magnetization transfer (ihMT) MRI contrast for preclinical studies using dipolar relaxation time ( T 1D ) filtering. NMR Biomed 2017;30:e3706.
11. Mchinda S, Varma G, Prevost VH, et al. Whole brain inhomogeneous magnetization transfer (ihMT) imaging: Sensitivity enhancement within a steady-state gradient echo sequence. Magn Reson Med https://doi.org/10.1002/mrm.26907.
Figures
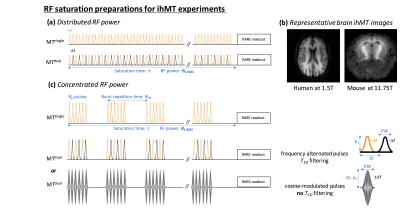
Fig.1. RF saturation preparations for ihMT experiments.
(a) Saturation using equally distributed RF pulses over the saturation period, τ, deposing a RF power B1,RMS. (b) Representative human (at 1.5T) and mouse (at 11.75T) brain ihMT images obtained with this saturation scheme. (c) Saturation using concentrated bursts of Np RF pulses, separated by relaxation periods, allowing an increase of the ihMT signal (ihMT boost effect). Dual frequency-offset saturation can be achieved either by frequency-alternated pulses (power, b1), which allow T1D-filtering or by cosine-modulated pulses (power, √2.b1), which allow simultaneous saturation at ±Δf.
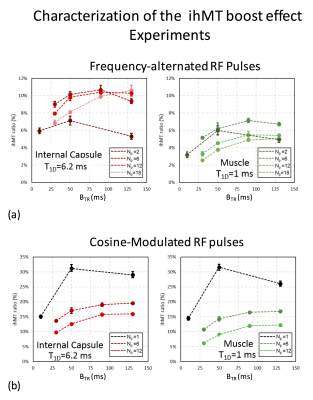
Fig.2. Characterization of the ihMT boost effect: experiments.
The maximum boost effect for dual frequency-offset saturation achieved by frequency alternated RF pulses (a) was obtained for {Np,BTR}={6,90ms} representing sensitivity gains of ~1.6 for internal capsule (ihMTR 6%->10.5%) and ~3 for muscle (ihMTR 2.3%->7%). This gain difference highlights the T1D dependence of the ihMT boost effect. For cosine-modulated RF pulses (b) a stronger sensitivity gain was obtained, with ihMTR~30% in both internal capsule and muscle for the most concentrated power configuration, Np=1. Conversely, excessive power concentration led to the loss of the ihMT boost effect with frequency-alternated pulses for internal capsule (Np=2, (a)).
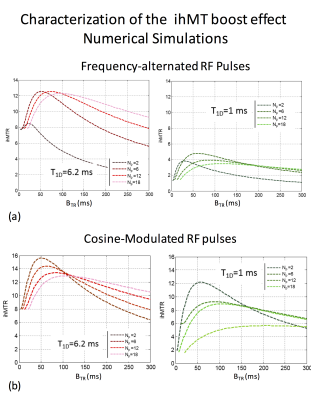
Fig.3. Characterization of the ihMT boost effect: Numerical simulations.
Simulated variations of ihMTR with {Np, BTR} obtained in short-T1D (1ms) and long-T1D (6.2ms) components show good general agreement with experiments for dual offset saturation achieved with both frequency alternated RF pulses (a) and cosine-modulated RF pulses (b). Of particular interest, the fundamental difference between dual offset saturation approaches in the most concentrated RF power configuration (Np=2) was predicted by the theory. Note that similarly to experiments, the loss of ihMT boost effect with Np=2 in (a) was less pronounced for short-T1D muscle.
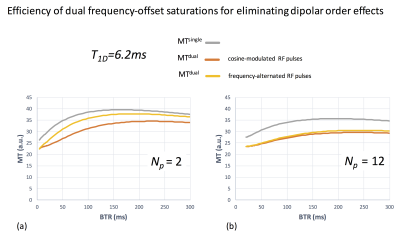
Fig. 4. Efficiency of dual frequency-offset saturations for eliminating dipolar order effects
Concentrating the RF power by increase of BTR and/or decrease of Np required to increase the b1 of individual RF pulses. High b1 values required for Np=2 (a) created strong dipolar order effects, which were only partially eliminated with the frequency-alternated dual saturation as evidenced by MTdual tending towards MTsingle (yellow curve). The Np=12 configuration (b) required lower b1, leading to weaker dipolar order effects and to better efficiency of the dual saturation. Simultaneous dual saturation achieved with cosine-modulated approach allowed efficient elimination of dipolar order effects regardless of b1 (a,b, orange curve).
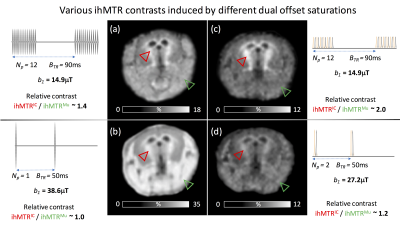
Fig.5. Various ihMTR contrasts induced by different dual offset saturations.
The different properties of dual frequency-offset saturation schemes were used to vary the ihMT contrast. High RF power concentration combined with cosine-modulated pulses allowed increasing the ihMT signal of short-T1D muscle and reducing the relative contrast between internal capsule and muscle (a,b). Higher specificity for long-T1D myelinated structures can be obtained by reduction of the RF power concentration and by using frequency-alternated RF pulses (Np=12) (c). High RF power concentration (Np=2) associated with frequency-alternated RF pulses (d) is counterproductive for both ihMT signal intensity and contrast between structures.