0552
Contrast Enhancement for Early Tumor Detection by Active-Feedback Field Locking and Refocusing1Chemistry & Biochemistry, University of California, Los Angeles, Los Angeles, CA, United States
Synopsis
Early detection of high-grade malignancy using novel contrast mechanisms and detection methods significantly increases the treatment options and the patients’ survival rate. For this purpose, the local magnetic-field-gradient variations due to irregular water contents and deoxyhemoglobin concentration in early glioblastoma multiforme (GBM) is sensitively detected by active-feedback electronic devices and nonlinear spin dynamics. The active-feedback phases and strength were tuned to manipulate the underlying spin dynamics and thus optimize the feedback-induced avalanching spin amplification effect. In vivo results from GBM mouse models show that up to 12 times of improved tumor-to-normal-tissue contrasts can be achieved to highlight early GBM.
Introduction
Contrast is a limiting factor for the early diagnosis of Glioblastoma multiforme (GBM). To amplify the small magnetic differences in MR parameters and thus generate contrast between tissues with only slight physiological variations, a non-linear approach by adding an active-feedback field into the system has been introduced [1]–[7]. In addition to the sensitivity to small variations between tissues of interest, it is necessary to address the pronounced field inhomogeneity when performing in vivo imaging. Here we propose and demonstrate two novel-designed feedback pulse sequences that employ active-feedback phases to further enhance the sensitivity to subtle tissue variations and to target the challenges of field inhomogeneity, leading to contrast enhancement. In vivo results of GBM animal model attained at a 7T MRI show that up to 12 times improved tumor-to-normal-tissue (TN) contrasts, compared with conventional MRI sequences, can be achieved to highlight tumor at an early stage that was not discernable after the injection of gadolinium (Gd)-based contrast agents.
Methods
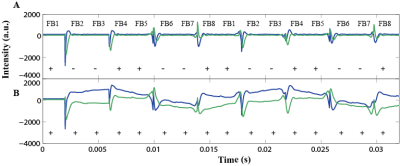
Dephasing of the transverse magnetization under field inhomogeneity results in a reduction of active-feedback field strength and thus loss of sensitivity to small resonance frequency differences between tissues, especially in in vivo environment [8]. Utilizing our home-built feedback hardware, we exploit the tunable phase and strength of the active-feedback fields to manipulate the underlying spin dynamics and thus adjust the feedback-induced avalanching spin amplification effect. Sequence optimization and field inhomogeneity minimization were performed through the newly designed π-train active-feedback pulse sequences (Fig. 1) by inserting four π pulses into feedback fields during the spin preparation time. The interplay of active-feedback fields and applied π pulses was then studied under two distinct sets of active-feedback phases (Fig. 1). Experiments were performed to analyze spin magnetization evolving under the two different sets of active-feedback phases (Fig. 2). In vivo experiments on mice at 7T MRI were carried out to demonstrate contrast enhancement as a result of optimally sustained magnetization and robustness to field inhomogeneity under tuned spin amplification effect and the applied π-train active-feedback pulse sequences (Fig. 3).Results
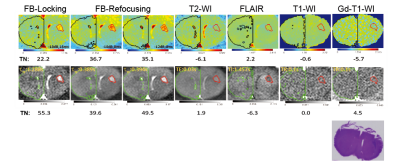
π-train active-feedback pulse sequences were designed to reinforce spin amplification effect by employing active-feedback phases and directing the magnetization toward the longitudinal axis to alter feedback-enhanced contrast for in vivo imaging. Fig. 2 shows that transverse magnetizations evolve differently under "FB-Locking" (Fig. 2A) and "FB-Refocusing" (Fig. 2B) pulse sequence, indicating distinct contrast mechanisms were provided by adjusting active-feedback phases. These pulse sequences aid in optimizing active-feedback field strength and active-feedback period (τFB and/or τTF) for contrast enhancement. TN contrasts were determined to show that the novel π-train active-feedback pulse sequences significantly improve the early tumor contrast when compared with conventional imaging methods. An isointense solid tumor was observed in both conventional T1-weighted and T2-weighted images and FLAIR shows a hypointense solid tumor with a small TN contrast (Fig. 3). Contrast enhancement at 7T MRI under both FB-Locking and FB-Refocusing pulse sequences highlighted the tumor region of the GBM mouse brain slice that was not apparent in the corresponding Gd-T1-weighted image, which was further confirmed by the dramatic change in TN contrast (Fig. 3).Discussion
FB-locking pulse sequence exploits the active-feedback field to lock the spin magnetization in the longitudinal direction, where the artifacts produced when magnetization appears at the transverse plane can be minimized. Such contrast mechanism is hypothesized to use the inserted π pulses to selectively excite spin magnetization in different tissue components, resulting in different spin relaxations and enhanced contrast. FB-refocusing pulse sequence utilizes active-feedback phases to push the spin magnetization toward the transverse plane, where along the way the spin magnetization projected on the transverse plane is refocused by a sequence of π pulses. Local sources of field variation such as abnormal blood vessel development in and around the tumor brought about the shift in local magnetic susceptibility and precession frequency. Due to such resonance mismatch, spin magnetization corresponding to tumor region were left behind at –z direction with a slower decay time, leading to a highlighted region in the images.Conclusion
The π-train active-feedback pulse sequences presented here establish novel spin dynamics to enhance in vivo contrast of tumors at early stages. In this new approach, manipulation of the strength and/or phase of the active-feedback fields between the inserted π pulses bolsters the magnetization evolution that yields maximal contrast enhancement and demonstrates robustness to field inhomogeneity. Experimental results also suggested that both contrast mechanisms, designed in the hope of preserving sensitive detection of small susceptibility, contribute to the improved contrast and may facilitate early detection of the tumor tissues.Acknowledgements
The authors gratefully acknowledge NSF, NSF-EAPSI program, MOST, SIT for the research funding.References
[1] S. Y. Huang, S. M. Wolahan, G. W. Mathern, D. J. Chute, M. Akhtari, S. T. Nguyen, M. N. Huynh, N. Salamon, and Y. Lin, “Improving MRI Differentiation of Gray and White Matter in Epileptogenic Lesions Based on Nonlinear Feedback,” vol. 786, pp. 776–786, 2006.
[2] S. Datta, S. Y. Huang, Y. Lin, S. Datta, S. Y. Huang, and Y. Lin, “The transient dynamics leading to spin turbulence in high-field solution magnetic resonance : A numerical study The transient dynamics leading to spin turbulence in high-field solution magnetic resonance : A numerical study,” Chem Phys, vol. 154501, 2006.
[3] Y. Lin, N. Lisitza, S. Ahn, and W. S. Warren, “Resurrection of Crushed Magnetization and Chaotic Dynamics in Solution NMR Spectroscopy,” Science (80-. )., vol. 290, pp. 118–121, 2000.
[4] S. Y. Huang, A. V. A. P. Chung, and Y. Lin, “Visualizing Contrast in Magnetic Resonance Imaging,” Concepts Magn Reson, vol. 30A, pp. 378–393, 2007.
[5] S. Datta, S. Y. Huang, and Y. Lin, “Understanding Spin Turbulence in Solution Magnetic Resonance Through Phase Space Dynamics and Instability,” Concepts Magn Reson, vol. 28A, pp. 410–421, 2006.
[6] S. Datta, S. Y. Huang, and Y. Lin, “Contrast Enhancement by Feedback Fields in Magnetic Resonance Imaging †,” J Phys Chem B, vol. 110, pp. 22071–22078, 2006.
[7] S. Y. Huang, S. S. Yang, and Y. Lin, “Sensitivity of Feedback-Enhanced MRI Contrast to Macroscopic and Microscopic Field Variations,” Magn Reson Med, vol. 61, pp. 925–936, 2009.
[8] S. Y. Huang, D. W. Hwang, L. Hwang, and Y. Lin, “Designing active feedback-based contrast enhancement for in vivo imaging,” Magn Reson Mater Phy, 2006.
Figures