0539
In-vivo Vagus Nerve to Central Nervous System Tracing using Manganese Enhanced Magnetic Resonance Imaging1Biomedical Engineering, Purdue University, West Lafayette, IN, United States, 2Electrical and Computer Engineering, Purdue University, West Lafayette, IN, United States
Synopsis
Tracing neuronal connections of the peripheral and central nervous system has relied on invasive techniques that make it difficult to reconstruct information. We demonstrate the feasibility of utilizing manganese enhanced magnetic resonance imaging (MEMRI) and vagus nerve stimulation (VNS) to trace the vagus nerve to central nervous system (CNS) connections. This experimental approach shows the non-invasive visualization and quantified increased enhancement of manganese transport from the nodose ganglion to the left nucleus tractus solitaries (NTS).
Purpose
It has been shown that manganese (Mn2+) can be utilized as a neuronal tracer due to its excellent T1 contrast and its ability to be transported by neurons1. We aimed to utilize Mn2+ to trace the vagal projection into the brain. The rationale is that vagus nerve stimulation (VNS) allows for the activated cells to selectively uptake Mn2+ through calcium channels, and travel along axons via microtubule-based transport. Mn2+ can then be released at the synapse and up-taken by the post-synaptic neuron. In this study, we have developed a novel experimental approach using manganese enhanced magnetic resonance imaging (MEMRI) to demonstrate the feasibility of visualizing the vagus nerve to central nervous system (CNS) circuity within the rat model.Method
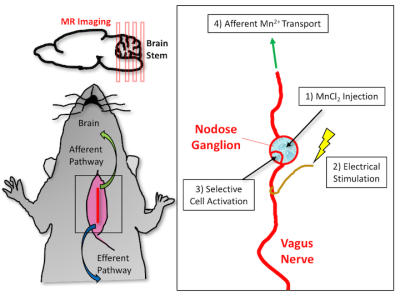
Four, male, Sprague-Dawley (SD) rats (n=4, 240-310g) were used in this study. All rats were weighed and anesthetized before MR imaging with 5% Isoflurane (500 ml/min) for 5 minutes. Anesthetization was maintained throughout MR scanning at 2% anesthesia level (500 ml/min). Respiratory rate and temperature were monitored and controlled throughout imaging. The rats were placed prone in a custom rat holder that was designed in-house with their noses positioned in a nose cone. Motion was minimized through the use of a bite bar and ear bars. A surface coil was placed on top of the head and fastened down before being scanned in a 7T small-animal MRI system (BioSpec 70/30, Bruker). A 3D, T1 RARE sequence with no gaps was used to obtain T1-weighted brainstem images (TR/TE = 300/10ms; FA = 90°; matrix size = 192 x 192 x 64; FOV = 32 x 32mm x 28mm; four averages). After the first imaging session, the rats underwent surgery for implantation of a bipolar electrode at the left cervical vagus nerve. The surgery consisted of first exposing the nodose ganglion along the vagus nerve. After exposing this injection site, the electrode was implanted, and 1.5μl of 500mM MnCl2 was injected. The rats were then stimulated with electrical pulses, used from previous preclinical studies (biphasic square pulses with inter-pulse duration (IPD) = 50 ms; pulse amplitude (PA) = 1mA; pulse width (PW) = 0.5ms; frequency = 5Hz; 20 seconds on and 40 seconds off), for four hours (Fig. 1). Once the four-hour post-injection period was finished, all rats were imaged again under the same protocol. Images were compared to each other to visualize the progression of the Mn2+ transport. Normalized signal intensity was calculated from the images, and percent increase in enhancement relative to the pre-contrast images was quantified. The local signal intensity in the left NTS was normalized to an easily identifiable region of interest at a similar depth from the coil.Results
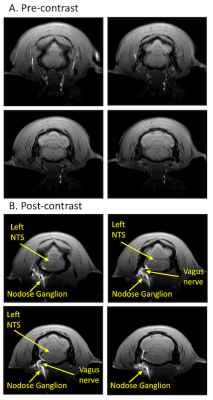
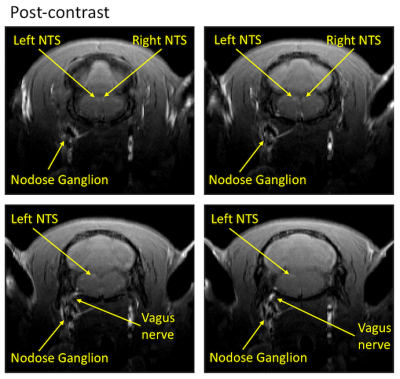
This experimental methodology has demonstrated the ability to trace neuronal tracts of the vagus nerve (10th cranial nerve, 10n) to CNS with MEMRI. When we compared pre-injection images of the rat brain with the corresponding post-injection images, it was apparent that manganese was transported from the nodose ganglion to the left nucleus tractus solitaries (NTS) (Fig. 2). Additionally, there was an increase in normalized signal intensity of the left NTS post-contrast (21.88% increased enhancement relative to pre-contrast). This effect was observed at the 4-hour post-injection time. One of the stimulation rats showed a bilateral enhancement of the left NTS and right NTS (Fig. 3), suggesting the transport of Mn2+ across a synapse (from the left NTS to the right NTS).Conclusion
We have shown that the use of MEMRI to assess the Mn2+ transport along peripheral nerves is feasible. We developed a robust, non-invasive, contrast-enhanced experimental MRI approach to trace neuronal connections in-vivo over time. This protocol overcomes the challenge of other neuronal tracers that require the sacrifice, sectioning of the brain, and reconstruction of slices to gather information1,2. These results demonstrate the feasibility of using MEMRI to visualize vagus-CNS connections in the alive rat.Acknowledgements
No acknowledgement found.References
1. Pautler RG, Silva AC, Koretsky AP. In vivo neuronal tract tracing using manganese-enhanced magnetic resonance imaging. Magn Reson Med. 1998;40(5):740-748. doi:10.1002/mrm.1910400515.
2. Pautler RG. In vivo, trans-synaptic tract-tracing utilizing manganese-enhanced magnetic resonance imaging (MEMRI). NMR Biomed. 2004;17(8):595-601. doi:10.1002/nbm.942.
Figures