0534
Pushing the limits of short-T2 MRI: 200 mT/m gradient strength and 2 MHz bandwidth1Institute for Biomedical Engineering, ETH Zurich and University of Zurich, Zurich, Switzerland
Synopsis
MRI of tissues with short transverse relaxation times below 1 millisecond such as bone or myelin raises both scientific and clinical interest. However, achieving high spatial resolution for short-T2 signals is challenging as large gradient strengths are required. Furthermore, large G implies high signal bandwidth, thus increasing the demands for short-T2 imaging techniques. Therefore, currently, short-T2 imaging faces significant restrictions with respect to spatial resolution and accessible T2s. In this work, all these challenges are tackled to expand the limits of short-T2 MRI using large G up to 200 mT/m and high BW up to 2 MHz.
Introduction
MRI of tissues with short transverse relaxation times (T2 or T2*) below 1 millisecond such as bone or myelin raises both scientific and clinical interest1. However, achieving high spatial resolution (dr) for short-T2 signals is challenging as large gradient strengths G $$$\sim$$$ 1/(dr·T2) are required2.
Furthermore, large G implies high signal bandwidth (BW), thus increasing the demands for short-T2 imaging:
- Large central k-space gaps associated with the initial RF dead time in zero-echo-time (ZTE)-based techniques2,3
- High-BW RF excitation
- High sensitivity to rapidly decaying spurious signals
Moreover, some of these requirements are particularly difficult to fulfill for human MRI scanners. Therefore, currently, short-T2 imaging faces significant restrictions with respect to spatial resolution and accessible T2s. In this work, all the above challenges are tackled to expand the limits of short-T2 MRI using large G up to 200 mT/m and high BW up to 2 MHz for both phantom and human in-vivo scanning.
Methods
MRI of ultra-short T2s at high resolution was implemented by means of:
- A high-performance gradient providing 200 mT/m strength with 600 mT/m/ms slewrate at unrestricted duty cycle4.
- The 3D PETRA technique combining ZTE and single-point imaging (SPI) data where the SPI plateau serves to fill the dead-time gap and improves resolution5.
- High-BW sweep excitation and corresponding reconstruction6,7.
- Elimination of background signal by using 1H-free loop and birdcage RF coils8,9.
- Elimination of transients in transmit-receive (T/R) switches10 and from RF amplifier
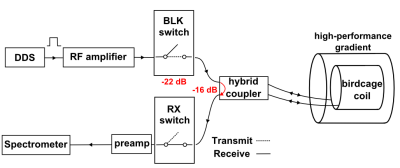
Measurements were performed in a 3T Achieva MRI system (Philips Healthcare, Best, Netherlands) complemented with a custom-made RF chain (Figure 1a).
Results
Figure 2 illustrates the challenge arising from RF amplifier ring-down in high-BW MRI. With both amplifiers the ring-down creates a serious artifact in the images which is stronger for amplifier A and increases with power and shorter readouts. By providing improved attenuation with an additional blanking switch in the transmit path, artifact-free images are obtained.
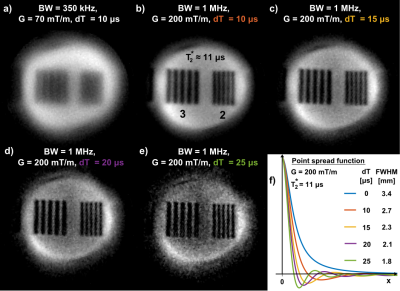
Increasing spatial resolution in short-T2 imaging is demonstrated in Figure 3 using a PMMA sample with T2* ≈ 11 μs. The basis for resolving fine structures is laid by increasing G from 70 to 200 mT/m and hence BW from 350 kHz to 1 MHz (Figure 3a-b). Further improvements are achieved by additionally increasing the SPI part in PETRA data which originally serves for filling the central k-space gap for dead time dT (Figure 3b-e). With dT growing from 0 to the acquisition duration, the PSF moves from a Lorentzian to a Sinc lineshape with thinner main lobe3, hence improving resolution, yet at the price of increased scan time and reduced SNR. In this way, ultra-fast relaxing components can be resolved with 1.8 mm resolution.
Applying the above approach to short-T2 imaging of a bone sample (Figure 4), shows that at clinical gradient strength spatial resolution is insufficient to capture relevant microstructure (Figure 4b). Furthermore, the ultra-short T2 component visible at small dead time is strongly blurred. However, a 5-fold increase of G to 200 mT/m and using PETRA with dT = 20 μs allows significant improvement in image quality, enabling the discrimination of sub-millimeter microstructure of the trabecular bone (Figure 4c). The ultra-short T2 component is sharpened but also reduced in amplitude due to larger dT.
Feasibility of MRI with gradients up to 200 mT/m and ultra-high bandwidth up to 2 MHz in humans is demonstrated in Figure 5. The head image (Figure 5a) shows mainly proton density with clear depiction of tissue-air interfaces and considerable signal in bone and teeth. In the wrist (Figure 5b), additional T1 contrast emphasizes bone marrow. Water-fat interfaces are sharply depicted without off-resonance artifacts typical for radial images.
Discussion and Conclusion
In this work, we demonstrated the feasibility of MRI of signals with ultra-short T2s at high resolution and its applicability in human-sized scanners. This was enabled by using dedicated hardware and specifically adapted methodology. In particular, additional rapid RF amplifier unblanking was employed to avoid serious image artifacts from ring-down with time constants similar to the targeted T2s.
In this way, extreme combinations of T2 and resolution (11 μs/1.8 mm, 200 μs/0.46 mm) were obtained. In vivo application of such protocols was shown to be feasible and is expected to benefit from suppression of long-T2 tissues7,11.
A principal challenge of imaging ultra-short T2s is a low SNR efficiency of the required high-bandwidth acquisition schemes. Possible improvements are anticipated from using coil arrays instead of birdcage or single-loop surface coils. Furthermore, the time-consuming SPI part in PETRA may be replaced by more efficient encoding approaches12.
Acknowledgements
No acknowledgement found.References
1. Graeme M. Bydder, Gary D. Fullerton, Ian R. Young. MRI of Tissues with Short T2s or T2*s. Wiley; 2012.
2. Weiger M, Pruessmann KP. MRI with Zero Echo Time. eMagRes. 2012;1:311–322.
3. Froidevaux R, Weiger M, Brunner DO, Wilm BJ, Dietrich BE, Pruessmann KP. Filling the dead time gap in zero echo time MRI : principles compared. Magn Reson Med. 2017; DOI 10.1002/mrm.26875
4. Weiger M, Overweg J, Rösler MB, Froidevaux R, Hennel F, Wilm BJ, Penn A, Sturzenegger U, Schuth W, Mathlener M, et al. A high-performance gradient insert for rapid and short-T2 imaging at full duty cycle. Magnetic Resonance in Medicine. 2017. DOI 10.1002/mrm.26875
5. Grodzki DM, Jakob PM, Heismann B. Ultrashort echo time imaging using pointwise encoding time reduction with radial acquisition (PETRA). Magnetic Resonance in Medicine. 2012;67:510–518.
6. Schieban K, Weiger M, Hennel F, Boss A, Pruessmann KP. ZTE Imaging With Enhanced Flip Angle Using Modulated Excitation. 2014; 74:684–693.
7. Li C, Magland JF, Zhao X, Seifert AC, Wehrli FW. Selective In Vivo Bone Imaging with Long-T2 Suppressed PETRA MRI. 2016; 77:989–997.
8. Rösler MB, Weiger M, Brunner DO, Schmid T, Pruessmann KP. An RF birdcage coil designed for an insert gradient coil dedicated to short-T2 MRI. In Proceedings of the 26th Annual Meeting of ISMRM, Honolulu. 2017:2668.
9. Rösler MB, Weiger M, Schmid T, Brunner DO, Froidevaux R PK. Ultrasonic soldering on glass for the construction of MRI coils with minimized background signal in short-T2 images. In Proceedings of the 33rd Annual Scientific Meeting of ESMRMB, Vienna, Austria. 2016:87.
10. Brunner DO, Furrer L, Weiger M, Baumberger W, Schmid T, Reber J, Dietrich BE, Wilm BJ, Froidevaux R, Pruessmann KP. Symmetrically Biased T/R Switches for NMR and MRI with Microsecond Dead Time. Journal of Magnetic Resonance. 2016;263:147–155.
11. Lee H, Weiger M PK. High resolution dual-echo subtraction ZTE imaging at 7T with TE estimation based on multiple scaled trajectories. In Proceedings of the 25th Annual Scientific Meeting of ISMRM, Honolulu, Hawaii, USA. 2017:4023.
12. Froidevaux R, Weiger M, Pruessmann KP. Zero echo time imaging with hybrid gap filling Zero Echo Time MRI ( ZTE ). In Proceedings of the 34th Annual Scientific Meeting of ESMRMB, Barcelona, Spain. 2017:71
13. Dietrich BE, Brunner DO, Wilm BJ, BarmeT C, Gross S, Kasper L, Haeberlin M, Schmid T, Vannesjo SJ, Pruessmann KP. A field camera for MR sequence monitoring and system analysis. Magnetic Resonance in Medicine. 2016;75:1831–1840.
Figures