0530
Prenatal Maternal Cortisol Response Predicts One-month Infant White Matter Microstructure1Waisman Center, University of Wisconsin Madison, Madison, WI, United States, 2Psychology, University of Wisconsin Madison, Madison, WI, United States, 3Center for Healthy Minds, University of Wisconsin Madison, Madison, WI, United States, 4Psychiatry, University of Wisconsin Madison, Madison, WI, United States, 5Medical Physics, University of Wisconsin Madison, Madison, WI, United States
Synopsis
Exposure to differing concentrations of cortisol likely has a significant impact on brain development in childhood and adolescence; however, little is known about the time immediately following birth. Using multi-shell diffusion imaging data, we examined the associations between prenatal maternal diurnal cortisol patterns and infant white matter microstructure. Infant measures were associated with the slope of the maternal cortisol response across white matter, suggesting variations of cortisol within the intrauterine environment may have a significant influence on processes of early brain development.
Introduction
The human brain undergoes rapid but systematic development that results from a cascade of intricate processes governed by sensitive interactions between genetic and environmental factors1,2. Increasing evidence suggests that early life experiences play a critical role in early child development and that genetic and environmental alterations can have long lasting consequences for fetal and postnatal brain maturation3,4. One factor that may significantly impact the intrauterine environment and consequently influence early brain development is exposure to heightened concentrations of the human stress hormone, cortisol. Cortisol has wide-ranging metabolic and immune effects5, while also playing a necessary role in promoting processes of brain development, including neurogenesis, synaptogenesis, axonal and dendritic arborization, and myelination6,7. Despite maternal cortisol increasing 3-5-fold over the course pregnancy8, little is known about how variations of maternal cortisol concentrations or rhythms impact early brain development. Utilizing salvia samples collected from mothers during the third trimester of pregnancy and neuroimaging data acquired in their 1-month old infants, we examined the associations between prenatal maternal diurnal cortisol rhythms and infant white matter microstructure.Methods
Mothers were identified and enrolled during the second trimester of pregnancy. Mothers collected their own saliva and recorded the collection times in the home three times a day (upon waking, in the afternoon, and right before bed) for three consecutive days at approximately 35 weeks gestation. Upon assaying salvia samples, maternal diurnal cortisol curves were estimated using linear mixed effects models. At one month of age, infants underwent non-sedated MRI, which included multi-shell diffusion imaging. After standard processing, including correction of motion and eddy currents and non-parenchyma tissue removal, data were fit to the diffusion tensor model using RESTORE9 and the neurite orientation dispersion and density imaging (NODDI) model10. Fractional anisotropy (FA), and mean and radial diffusivity (MD, RD, respectively), as well as the intra-cellular volume fraction (vIC ) and orientation dispersion index (ODI) were calculated and aligned to a study-specific template created using ANTs11. Spatially aligned parameter maps were smoothed with a 5mm full-width-at-half-maximum kernel. Voxelwise associations between 35-week diurnal slope and infant white matter microstructure (FA, MD, RD, AD, vIC, and ODI) were assessed using nonparametric permutation testing (randomise12) with 5000 permutations and threshold-free cluster enhancement13. Additional nuisance covariates of infant age (corrected to a 40-week gestation), 35-week diurnal intercept, maternal education level and total infant motion were included.Results
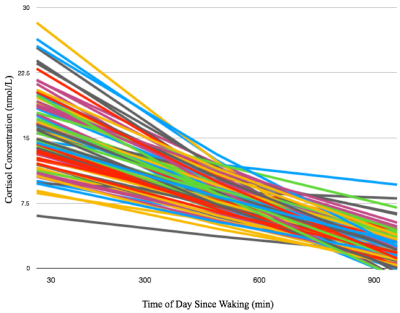
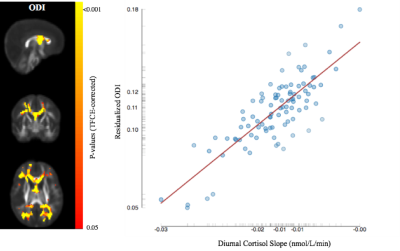
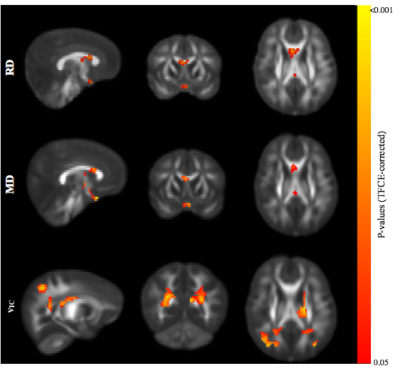
Representative cortisol curves for each participating mother are shown in Fig.1. The intercepts and slopes of these curves vary between individual mothers, illustrating the significant individual variation of the cortisol response. We observed highly significant positive associations between 35-week maternal cortisol slopes and DTI/NODDI indices across the brain. In particular, ODI was positively associated with maternal cortisol slope across white matter regions (Fig 2) and including regions such as the genu and body of the corpus callosum, optic radiations and frontal white matter. These associations indicate smaller amounts of dispersion or fanning in association with more negative cortisol slopes. Similarly, MD and RD in the corpus callosum were positively correlated with maternal cortisol slope (Fig 3), while higher vIC in the superior longitudinal fasciculus, thalamic radiations, parietal and occipital white matter was associated with higher cortisol slope.Conclusions
Prenatal maternal cortisol patterns are associated with alterations to 1-month infant white matter microstructure. In particular, higher ODI, MD and RD in infants born to mothers whose cortisol slopes are flatter across the day are consistent with reductions of microstructural integrity and suggestive of disrupted or immature white matter development. These results provide new insights into understanding patterns of white matter microstructure and add to the growing consensus that prenatal life experiences have an important role on early brain development. Future analyses will examine whether these relationships differ in male and female infants as well as how these early alterations may impact later childhood development.Acknowledgements
We thank the participants and their families. We also thank Corrina Frye, Corey Schmidt, Nicole Schmidt, and Sarah Short for their help. This work was supported by the National Institutes of Mental (P50 MH100031, RJD, R01 MH101504, HHG, PI, and K99MH110596, DCD, PI). Infrastructure support was also provided, in part, by a core grant to the Waisman Center from the National Institute of Child Health and Human Development (U54 HD090256).References
1. Dubois J, Dehaene-Lambertz G, Kulikova S, Poupon C, Hüppi PS, Hertz-Pannier L. The early development of brain white matter: A review of imaging studies in fetuses, newborns and infants. Neuroscience 276:48–71
2. Stiles J, Jernigan TL. The Basics of Brain Development. Neuropsychology Review 20:327–348
3. Davidson RJ, McEwen BS. Social influences on neuroplasticity: stress and interventions to promote well-being. Nature Neuroscience 15:689–695
4. Entringer S, Buss C, Wadhwa PD. Prenatal stress, development, health and disease risk: A psychobiological perspective-2015 Curt Richter Award Paper. Psychoneuroendocrinology 62:366–375
5. Chrousos GP, Kino T. Glucocorticoid signaling in the cell. Expanding clinical implications to complex human behavioral and somatic disorders. Ann. N. Y. Acad. Sci. 2009;1179:153–66
6. Davis EP, Sandman CA. The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Dev 2010;81:131–48
7. Davis EP, Head K, Buss C, Sandman CA. Prenatal maternal cortisol concentrations predict neurodevelopment in middle childhood. Psychoneuroendocrinology 2017;75:56–63
8. Sandman CA, Glynn L, Schetter CD, Wadhwa P, Garite T, Chicz-DeMet A, Hobel C. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): priming the placental clock. Peptides 2006;27:1457–63
9. Chang L, Jones DK, Pierpaoli C. RESTORE: Robust estimation of tensors by outlier rejection. Magnetic Resonance in Medicine 53:1088–1095
10. Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. NeuroImage 61:1000–1016
11. Avants B, Epstein C, Grossman M, Gee J. Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Medical image analysis 12:26–41.
12. Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. NeuroImage 92:381–397
13. Smith S, Nichols T. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage 44:83–98
Figures