0523
Emerging Metabolic Trajectories in the Developing Fetal Brain1Developing Brain Research Laboratory, Children's National Health System, Washington, DC, United States, 2Diagnostic Imaging and Radiology, Children's National Health System, Washington, DC, United States, 3Pediatrics, The George Washington University School of Medicine, Washington, DC, United States
Synopsis
Knowledge of metabolite changes in the fetal brain will provide insight into the biochemical changes in the developing brain. Findings from measurement of metabolite concentration as a function of increasing GA are reported. Significant increases in NAA, Cr, Cho and scyllo-Inositol with increasing GA in the fetal brain was observed in this study. Changes in myo-Inositol with GA did not reach significance.
Introduction
Methods
157 healthy pregnant women were scanned during the second and third trimester of pregnancy with GA ranging from 18 to 39 weeks on a 1.5T GE scanner using protocols approved by our Institutional Review Board. 92 fetuses were scanned twice at a mean GA of 27(±4) and 35(±3) weeks of gestation at the first and second MRI scan, respectively. SSFSE images acquired in all three planes were used as a guide to place the voxels in the central brain of the fetal brain for spectroscopic measurements. Spectra were acquired using PRESS localization sequence (TE/TR: 144/1500 ms). 128-192 averages of water suppressed spectra were acquired along with 16 averages of water unsuppressed spectra from a 30x30x30 mm3 voxel placed in the central brain. Phase and frequency correction were performed using programs written in Matlab and spectra were quantified in the ‘LCModel’ (4) program using water as an internal reference. Data with CRLB >20% for total Choline (tCh) were excluded from further analysis. For all other metabolites, exclusion criteria included CRLB>100%. Given that NAA and Cre concentrations are lower at early GAs, exclusion criteria of CRLB>100% was used in this study which is higher than 20% that has traditionally been used to avoid biasing the metabolite concentrations to higher values (5). Statistical analysis included linear regression to assess metabolic trajectory as a function of GA and a paired t-test to study the trajectory within subject.Results
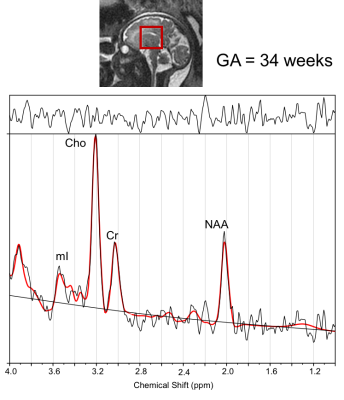
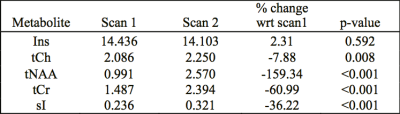
Figure 1 shows voxel placement and LCModel analysis result of a typical spectrum acquired from the fetal brain. NAA, Cre, Cho and mI were consistently measured in > 85% of the spectra. Table 1 shows averaged metabolite concentration during the two visits in pregnant women who underwent two sets of MRI scans. The preliminary results showed significantly increased NAA, Ch, Cre and scyllo-Inositol with increasing GA in both set of analyses. No significant difference was observed in mI concentration across different GAs. All fetuses had a structurally normal brain MRI study.Discussion
Our results are in keeping with existing literature describing metabolic changes in the fetal and neonatal brain studied at one time point (6,7). This is the first study to delineate metabolite changes within the same fetus as a function of in utero third trimester brain maturation. We also report for the first time on the presence and evolution of scyllo-inositol levels in the fetal brain. We currently have an ongoing validation study underway to evaluate the reproducibility of this metabolite measurement in the developing brain. Although the exact function of scyllo-inositol in the human brain is poorly understood it has been observed to be tightly coupled with mI concentration in the adult brain and could likely be involved in cellular growth. Extracting information about its levels and role in the immature developing warrants further study.Conclusion
This study demonstrates that high quality MRS data can be successfully acquired from the fetal brain with a success rate of 85%. Information about normative levels of metabolites and expected trajectory in a healthy developing brain may help identify the compromised fetus that is showing deviations from normal biochemical development in-utero and lead to better surveillance in the high-risk pregnancy.Acknowledgements
Supported by NIH R01HL116585-01References
1. Kreis R, Ernst T, Ross BD. Development of the human brain: In vivo quantification of metabolite and water content with proton magnetic resonance spectroscopy. Magnetic Resonance in Medicine 1993;30(4):424-437.
2. van der Knaap MS, van der Grond J, van Rijen PC, Faber JA, Valk J, Willemse K. Age-dependent changes in localized proton and phosphorus MR spectroscopy of the brain. Radiology 1990;176(2):509-515.
3. Horská A, Kaufmann WE, Brant LJ, Naidu S, Harris JC, Barker PB. In vivo quantitative proton MRSI study of brain development from childhood to adolescence. Journal of Magnetic Resonance Imaging 2002;15(2):137-143.
4. Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magnetic Resonance in Medicine 1993;30(6):672-679.
5. Kreis R. The trouble with quality filtering based on relative Cramér-Rao lower bounds. Magnetic Resonance in Medicine 2016;75(1):15-18.
6. Evangelou IE, du Plessis AJ, Vezina G, Noeske R, Limperopoulos C. Elucidating Metabolic Maturation in the Healthy Fetal Brain Using 1H-MR Spectroscopy. American Journal of Neuroradiology 2016;37(2):360-366.
7. Blüml S, Wisnowski JL, Nelson JMD, Paquette L, Gilles FH, Kinney HC, Panigrahy A. Metabolic Maturation of the Human Brain From Birth Through Adolescence: Insights From In Vivo Magnetic Resonance Spectroscopy. Cerebral Cortex 2013;23(12):2944-2955.
Figures