0521
Intravoxel Incoherent Motion Diffusion-weighted MRI for Assessing Necroinflammation in Patients with Chronic Liver Disease1Centre de recherche du centre hospitalier de l'Université de Montréal (CRCHUM), Montreal, QC, Canada, 2MR Clinical Science, Philips Healthcare Canada, Markham, ON, Canada, 3Department of Radiology, Radio-Oncology and Nuclear Medicine, Université de Montréal, Montreal, QC, Canada, 4Department of Gastroentology and Hepatology, Université de Montréal, Montreal, QC, Canada, 5Department of Medicine, Division of Gastroenterology, McGill University Health Centre (MUHC), Montreal, QC, Canada, 6Department of Pathology, Centre hospitalier de l'Université de Montréal (CHUM), Montréal, QC, Canada, 7Department of Pathology and Cellular Biology, Université de Montréal, Montreal, QC, Canada
Synopsis
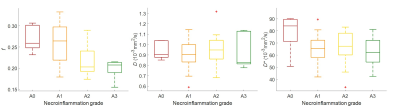
Necroinflammation is a hallmark feature in several causes of chronic liver disease. Because it has multiple tissue contrast mechanisms, MRI is ideally suited for characterization of histopathological changes (i.e. inflammation, fat, iron, and fibrosis) that may occur concomitantly in chronic liver disease. We evaluated intravoxel incoherent motion (IVIM) diffusion-weighted imaging (DWI) MRI for assessment of necroinflammation. Perfusion fractions were significantly correlated with necroinflammation grades (ρ = 0.49, P < 0.0001) and could discriminate grades ≤ A1 vs. ≥ A2 and ≤ A2 vs. A3 with good accuracy (AUC: 0.81 and 0.80, respectively). Our results suggest that perfusion fraction may be used for assessing liver necroinflammation.
Introduction
Necroinflammation causes liver damage leading to the activation of hepatic stellate cells and liver fibrosis in chronic liver disease.1 An animal study suggested that inflammation may reduce the perfusion fraction in rabbits with steatohepatitis compared to controls.2 Prior studies have also shown that perfusion fraction is correlated with fibrosis3,4,5,6 and with steatohepatitis7; diffusion coefficient is correlated with steatohepatitis4,7,8,9,10 and with fibrosis6,8; pseudo-diffusion coefficient is correlated with steatosis7,8 and with fibrosis.5,8 The purpose of this study was to evaluate the diagnostic accuracy of intravoxel incoherent motion (IVIM) diffusion-weighted imaging (DWI) magnetic resonance imaging (MRI) parameters for assessing histology-determined necroinflammation grades in patients with chronic liver disease.Materials and Methods
This cross-sectional review board-approved study included patients who underwent liver biopsy as part of their clinical standard of care for suspected or known chronic liver disease. Adult patients with hepatitis C virus infection, hepatitis B virus infection, nonalcoholic steatohepatitis, or autoimmune hepatitis were recruited between January 2014 and September 2017 at the hepatology clinics of the two participating institutions. IVIM was performed using a respiratory-triggered spin-echo diffusion-weighted echo-planar imaging sequence on a clinical 3.0 T system (Achieva TX, Philips Healthcare, Best, Netherlands) using a 16-channel body array for signal reception. Sequence parameters were: TR = 2000 ms, TE = 57 ms, 10 b values (0, 10, 20, 30, 40, 50, 100, 200, 400, 800 s/mm2), field-of view = 350 mm x 350 mm, in-plane resolution = 3.25 mm x 3.25 mm, slice thickness = 5mm, slice gap = 0.5mm, 30 slices, SENSE factor = 2, averages = 2, acquisition time of about 6 minutes (variable depending on the breathing rhythm). IVIM-DWI parameters (perfusion fraction – f, diffusion coefficient – D, and pseudo-diffusion coefficient – D*) were obtained using a least-squares non-linear regression and a segmented bi-exponential model. A region-of-interest comprising the liver over 5 central slices was used. Voxels showing a perfusion fraction (f) above 0.5 were discarded from the calculation since these voxels are mainly located in large vessels. Similarly, the 25% of the voxels showing the largest fit mean square error were also removed, to take into account that some voxels may be corrupted by residual breathing and cardiac motion. Necroinflammation grades, fibrosis stages, and steatosis grades were centrally scored by a liver pathologist. The pathologist was blinded to IVIM-DWI results. The image analyst was blinded to the biopsy results. IVIM-DWI parameters (f, D and D*) were investigated as potential biomarkers of liver necroinflammation, fibrosis, and steatosis. Spearman's correlation, Kruskal-Wallis test, Mann-Whitney U test, and receiver operating characteristic (ROC) analyses were performed. Bootstrapped 95% confidence intervals of area under ROC curves (AUC) were evaluated. Sensitivity, specificity, accuracy, positive predictive value, and negative predictive value corresponding to thresholds that maximized Youden's index were reported.Results
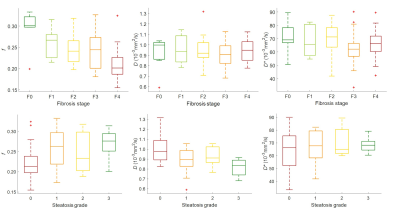
Sixty-six subjects were included. Table 1 summarizes the correlation between IVIM-DWI parameters and necroinflammation grade, fibrosis stage, and steatosis grade. Perfusion fractions and pseudo-diffusion coefficients were significantly different between all necroinflammation grades (P < 0.001 and P = 0.15, respectively). Perfusion fractions were significantly different between pairs of necroinflammation grades ≤ A1 vs. ≥ A2 (P = 0.01) and pseudo-diffusion coefficients between pairs of necroinflammation grades A0 vs. ≥ A1 (P = 0.04). Table 2 summarizes the performance of IVIM-DWI parameters for staging liver necroinflammation. Figure 1 shows a boxplot of IVIM-DWI parameters vs. necroinflammation grades. Figure 2 shows ROC curves of IVIM-DWI parameters vs. dichotomized necroinflammation grades. Figure 3 shows boxplots of IVIM-DWI parameters vs. fibrosis stages and steatosis grades.Conclusion
MRI is a multiparametric technique that allows quantification of biomarkers of liver chronic disease such as necroinflammation, fat, iron, and fibrosis. Perfusion fraction measured by IVIM-DWI MRI shows promise as a non-invasive technique for assessing liver necroinflammation. Diffusion and pseudo-diffusion coefficient showed poor diagnostic performance for assessing liver inflammation.Acknowledgements
This work has been supported by an Operating Grant from the Canadian Institutes of Health Research (CIHR)-Institute of Nutrition, Metabolism, and Diabetes (INMD) Operating Grant (#301520).
An Tang is supported by a Career Award from the Fonds de recherche du Québec en Santé and Association des Radiologistes du Québec (FRQS-ARQ #34939) and a New Researcher Startup Grant from the Centre de Recherche du Centre Hospitalier de l'Université de Montréal (CRCHUM).
References
1. Yin M, Glaser KJ, Manduca A, Mounajjed T, Malhi H, Simonetto DA, Wang R, Yang L, Mao SA, Glorioso JM, Elgilani FM, Ward CJ, Harris PC, Nyberg SL, Shah VH, Ehman RL. Distinguishing between Hepatic Inflammation and Fibrosis with MR Elastography. Radiology. 2017 Sep;284(3):694-705.
2. Joo I, Lee JM, Yoon JH, Jang JJ, Han JK, Choi BI. Nonalcoholic fatty liver disease: intravoxel incoherent motion diffusion-weighted MR imaging-an experimental study in a rabbit model. Radiology. 2014 Jan;270(1):131-40.
3. França M, Martí-Bonmatí L, Alberich-Bayarri Á, Oliveira P, Guimaraes S, Oliveira J, Amorim J, Gonzalez JS, Vizcaíno JR, Miranda HP. Evaluation of fibrosis and inflammation in diffuse liver diseases using intravoxel incoherent motion diffusion-weighted MR imaging. Abdom Radiol (NY). 2017 Feb;42(2):468-477.
4. Murphy P, Hooker J, Ang B, Wolfson T, Gamst A, Bydder M, Middleton M, Peterson M, Behling C, Loomba R, Sirlin C. Associations between histologic features of nonalcoholic fatty liver disease (NAFLD) and quantitative diffusion-weighted MRI measurements in adults. J Magn Reson Imaging. 2015 Jun;41(6):1629-38.
5. Ichikawa S, Motosugi U, Morisaka H, Sano K, Ichikawa T, Enomoto N, Matsuda M, Fujii H, Onishi H. MRI-based staging of hepatic fibrosis: Comparison of intravoxel incoherent motion diffusion-weighted imaging with magnetic resonance elastography. J Magn Reson Imaging. 2015 Jul;42(1):204-10.
6. Wu CH, Ho MC, Jeng YM, Liang PC, Hu RH, Lai HS, Shih TT. Assessing hepatic fibrosis: comparing the intravoxel incoherent motion in MRI with acoustic radiation force impulse imaging in US. Eur Radiol. 2015 Dec;25(12):3552-9.
7. Guiu B, Petit JM, Capitan V, Aho S, Masson D, Lefevre PH, Favelier S, Loffroy R, Vergès B, Hillon P, Krausé D, Cercueil JP. Intravoxel incoherent motion diffusion-weighted imaging in nonalcoholic fatty liver disease: a 3.0-T MR study. Radiology. 2012 Oct;265(1):96-103.
8. Parente DB, Paiva FF, Oliveira Neto JA, Machado-Silva L, Figueiredo FA, Lanzoni V, Campos CF, do Brasil PE, Gomes Mde B, Perez Rde M, Rodrigues RS. Intravoxel Incoherent Motion Diffusion Weighted MR Imaging at 3.0 T: Assessment of Steatohepatitis and Fibrosis Compared with Liver Biopsy in Type 2 Diabetic Patients. PLoS One. 2015 May 11;10(5):e0125653.
9. Leitão HS, Doblas S, Garteiser P, d'Assignies G, Paradis V, Mouri F, Geraldes CF, Ronot M, Van Beers BE. Hepatic Fibrosis, Inflammation, and Steatosis: Influence on the MR Viscoelastic and Diffusion Parameters in Patients with Chronic Liver Disease. Radiology. 2017 Apr;283(1):98-107.
10. Manning P, Murphy P, Wang K, Hooker J, Wolfson T, Middleton MS, Newton KP, Behling C, Awai HI, Durelle J, Paiz MN, Angeles JE, De La Pena D, McCutchan JA, Schwimmer JB, Sirlin CB. Liver histology and diffusion-weighted MRI in children with nonalcoholic fatty liver disease: A MAGNET study. J Magn Reson Imaging. 2017 Oct;46(4):1149-1158.
Figures