0516
Rapid multi-slice abdominal T1 mapping with high spatial and temporal resolution using an inversion recovery radial steady-state free precession (IR-radSSFP) technique1Electrical and Computer Engineering, The University of Arizona, Tucson, AZ, United States, 2Department of Medical Imaging, The University of Arizona, Tucson, AZ, United States, 3Biomedical Engineering, The University of Arizona, Tucson, AZ, United States, 4Siemens Healthcare, Tucson, AZ, United States
Synopsis
A radial IR steady-state free precession technique combined with a principal component based iterative algorithm are demonstrated for rapid high-resolution abdominal T1 mapping. This method yields high quality T1 maps for 10 slices with spatial resolution as high as of 0.83x0.83x3mm from data acquired in a breath-hold or a short free-breathing scan. The method is a significant improvement over existing abdominal T1 mapping techniques.
Introduction
Quantitative measurements of relaxation parameters is becoming a desired component for clinical diagnosis. T1 maps provide information to characterize neurological disorders [1,2,3], differentiate cirrhotic from healthy liver [4], diagnose declining kidney function [5], tumor malignancy [6], and myocardial infarction [7]. T1 maps are also used for perfusion quantification in dynamic contrast enhancement (DCE) MRI [8].
For abdominal imaging, the acquisition typically needs to fit within a breath-hold, limiting the amount of data acquired for T1 mapping. This affects the spatial and temporal resolution, signal-to-noise ratio (SNR) and/or slice coverage in T1 mapping. In fact, most techniques recently proposed for abdominal T1 mapping yield one slice per breath-hold [9,10,11,12], rendering the method inefficient when anatomical coverage is needed. For better slice coverage, a 3D stack-of-spirals technique with parallel imaging was recently demonstrated for T1 mapping of the abdomen in a breath-hold, but spatial resolution was limited to 2mm in plane [13].
Here we present an inversion-recovery (IR) radial balanced-SSFP (radSSFP) technique for multi-slice high resolution T1 mapping with high SNR. The method is demonstrated for breath-hold and free-breathing abdominal T1 mapping.
Technique and methods
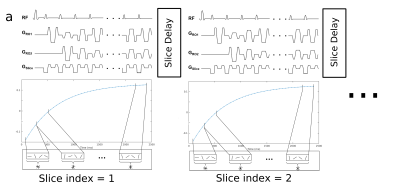
In the 2D IR-radSSFP pulse sequence data are continuously acquired following a 180° adiabatic inversion pulse using a train of constant flip angles and a radial k-space trajectory with tiny golden angle view ordering (Figure 1). All data for one slice are collected in a single shot during the recovery time of one inversion pulse (~3s) thus, minimizing the effects of motion. To generate k-space data sets at various inversion time (TI) points, data acquired in a single shot is partitioned into groups (with N=16 radial views per group) and the TI is approximated to the average TI of the views in the group.
Data for multiple slices are acquired by repeating the single shot acquisition. For breath-hold experiments, a slice-selective IR pulse combined with slice interleaving is used to minimize the slice delay and improve scan time efficiency (10 slices in ~20s breath-hold). For free-breathing experiments, a non-selective IR pulse is used to avoid slice cross-talk from out-of-plane motion; a slice delay of 5s allows spin recovery after the inversion pulse. Ten slices are acquired in ~1min free-breathing scan.
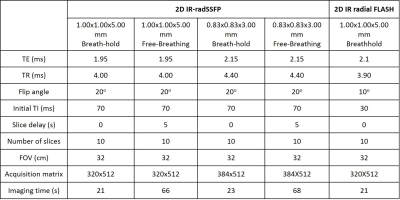
The IR-radSSFP sequence was implemented on a Siemens 3T Skyra scanner. Breath-hold and free-breathing experiments were performed at two spatial resolutions on 5 volunteers using a 32-elemenet body coil. The pulse sequence parameters are summarized in Table 1.
Because TI data sets are highly undersampled (4% relative to Nyquist) we use a principal component (PC) based algorithm to reconstruct the data [14]. The algorithm was implemented on 8 Nvidia Tesla P100 GPU using C++ with OpenCL. The algorithm recovers 5 PC images, which are converted into TI images. T1 is then fitted using the model in [15] with the algorithm from [16].
A reproducibility study was conducted by repeating the experiment twice. A Bland-Altman analysis was used to assess reproducibility.
Results and discussion
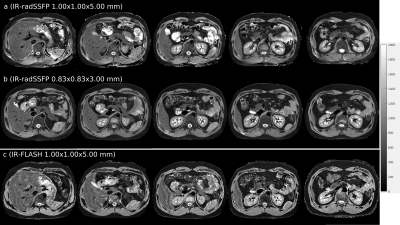
Figure 2 shows representative slices of (a) 1.00×1.00×5.00mm and (b) 0.83×0.83×3.00mm T1 maps obtained from breath-hold IR-radSSFP data, and (c) 1.00×1.00×5.00mm T1 maps obtained from an IR radial FLASH technique. Note the high anatomical detail and overall high quality of the T1 maps generated from IR-radSSFP data. The high spatial resolution and excellent SNR of these T1 maps provides sharp and conspicuous delineation of the kidney cortex and medulla, improved uniform mapping of the liver and spleen parenchyma with fewer ringing artifacts and better SNR than the IR-FLASH technique (both organs should be uniform in normal subjects). Note the smoother mapping of the vertebral bodies and discs with IR-radSSFP due to better SNR.
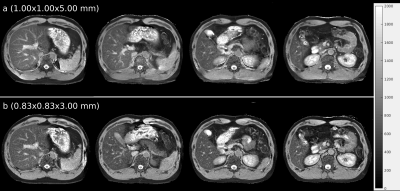
Figure 3 shows T1 maps from free-breathing IR-radSSFP; blood vessels show bright due to the non-selective IR pulse. Due to the fast imaging time (~3s/slice) the resulting T1 maps retain high quality, showing the potential of the technique for patients that cannot perform breath-holds.
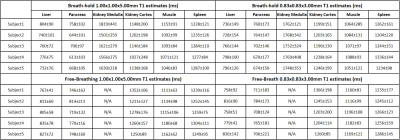
Table 2 shows the T1 values from different organs for 5 subjects. The values match literature reported values [17,18]. The coefficient of variation (CV) derived from a Bland-Altman analysis for the breath-hold experiments were 2.7% (1.00×1.00×5.00mm) and 2.5% (0.83×0.83×3.00mm). The CV for the free-breathing experiments at the 2 resolutions were 2.6% and 2.4%, respectively, showing a high degree of agreement in the mean T1 values from 2 separated experiments.
Conclusion
A fast multi-slice T1 mapping IR-radSSFP technique is proposed for high resolution abdominal imaging. T1 maps can be acquired within 3s/slice under breath-hold or free-breathing conditions. The proposed method is efficient and a significant improvement over existing clinical methods.Acknowledgements
The authors would like to acknowledge support from the Arizona Biomedical Research Commission (Grant ADHS14-082996) and the Technology and Research Initiative Fund (TRIF) Improving Health Initiative.References
[1] Woerman FG, Free SL, Koepp MJ, Ashburner J, Duncan JS. Voxel-by-voxel comparison of automatically segmented cerebral gray matter—a rater-independent comparison of structural MRI in patients with epilepsyc. NeuroImage. 1999;10:373-384.
[2] Larsson HB, Frederiksen J, Petersen J, Nordenbo A, Zeeberg I, Henriksen O, Olesen J. Assessment of demyelination, edema and gliosis by in-vivo determination of T1 and T2 in the brain of patients with acute attack of Multiple Sclerosis. Magn Reson Med. 1989;11:337–348.
[3] Williamson P, Pelz D, Merskey H, Morrison S, Karlik S, Drost D, Carr T, Conlon P. rontal, temporal and striatal proton relaxation times in schizophrenic patients and normal comparison subjects. Am J Psych. 1992;149:549-551.
[4] Kim KA, Park MS, Kim IS, Kim IS, et al. Quantitative evaluation of liver cirrhosis using T1 relaxation time with 3 Tesla MRI before and after oxygen inhalation. JMR. 2012;36:405-410.
[5] Huang Y, Sadowski EA, Artz NS, et al. Measurement and comparison of T1 relaxation times in native and transplanted kidney cortex and medulla. J Magn Reson Imaging. 2011;33:1241-1247.
[6] Goldberg MA, Hahn PF, Saini S, Cohen MS, Reimer P, Brady TJ, Mueller PR. Value of T1 and T2 relaxation times from echoplanar MR imaging in the characterization of focal hepatic lesions. AJR Am J Roentgenol. 1993;160(5):1011-1017.
[7] Polak JF, Vivaldi MT, Schoen FJ. Proton magnetic resonance of early myocardial infarction in rats. Invest Radiology. 1988;23:438-442.
[8] Steinhoff S, Zaitsev M, Zilles K, Shah NJ. Fast T1 mapping with volume coverage. Magn Reson Med. 2001;46:131-140.
[9] Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heartc. Magn Reson Med. 2004;52:141-146.
[10] Ma D, Gulani V, Seiberlich N, Liu K, Sunshine JL, Duerk JL, Griswold MA. Magnetic resonance fingerprintingc. Nature. 2013;495:187-192.
[11] Chen Y, Jiang Y, Pahwa S, Ma D, Lu L, Twieg MD, Wright KL, Seiberlich N, Griswold MA, Gulani V. MR fingerprinting for rapid quantitative abdominal imaging. Radiology. 2016;279(1):278-286.
[12] Wang X, Roeloffs V, Klosowski J, Tan Z, Voit D, Uecker M, Frahm J. Model-based T1 mapping with sparsity constraints using single-shot inversion-recovery radial FLASH. Magn Reson Med. 2017;00:00-00.
[13] Chen Y, Lee GR, Aandal G, Wright KL, Griswold MA. Rapid volumetric T1 mapping of the abdomen using three-dimensional through-time spiral GRAPPA. Magn Reson Med. 2015;75:1457-1465.
[14] Li Z, Bilgin A, Martin RD, Altbach IM. High resolution T1 map under 3 seconds using a PC based reconstruction and highly undersampled radial steady-state free-precession data. In: ISMRM; 2016; Singapore.
[15] Schmitt P, Griswold MA, Jakob PM, Kotas M, Gulani V, Flentje M, Haase A. Inversion recovery TrueFISP: quantification of T1, T2 and Spin Density. Magn Reson Med. 2004;51(4):661-667.
[16] Barral JK, Gudmundson R, Stikov N, Etezadi-Amoli M, Stoica P, Nishimura DG. A robust methodology for in vivo T1 mapping. Magn Reson Med. 2010;64:1057-1067.
[17] de Bazelaire CMJ, Duhamel GD, Rofsky NM, Alsop DC. MR imaging relaxation times of abdominal and pelvic tissues measured in vivo at 3.0 T: preliminary results. Radiology. 2004;230:652-659.
[18] Stanisz GJ, Odrobina EE, Pun J, Escaravage M, Graham SJ, Bronskill MJ, Henkelman RM. T1, T2 relaxation and magnetization transfer in tissue at 3T. Magn Reson Med. 2005;54:507-512.
Figures