0512
Metabolic concentrations in normal appearing brain tissue: 3 month after severe pediatric TBI.1Semenov Institute of Chemical Physics, Russian Academy of Sciences, Moscow, Russian Federation, 2Clinical and Research Institute of Emergency Pediatric Surgery and Trauma, Moscow, Russian Federation, 3Emanuel Institute of Biochemical Physics, Russian Academy of Sciences, Moscow, Russian Federation
Synopsis
In this study for the first time cerebral NAA, Asp and Glu concentrations of major inhibitory were simultaneously estimated in patients severe TBI . This work revealed significant reduction NAA and Asp concentrations in frontal lobe (on 65 and 61% respectively) in patients with severe TBI as compare to control group. At the same time, there was no significant change in Glu concentration. Our findings indicate that the main reason for the [NAA] reduction in the chronic severe TBI is caused by a decrease in [Asp]. Precursor of synthesis [NAA]. [Asp] reduction along with an unchanged Glu level may be caused by the dysfunction of one of the most important metabolic regulation systems - the malate-aspartate shuttle (MAS).
Introduction
Apart from structural changes, there are strong changes in cerebral metabolite concentrations after severe Traumatic brain injury. For example, many previous 1H MRS studies reported significant decrease of N-acetyl aspartate concentration ([NAA]), major neuronal marker, in different brain locations and time intervals from the injury. Possible reasons of NAA reduction is not still completely known. Disruption of NAA synthesis from Asp may result to its decrease. It’s became possible to measure simultaneously aspartate (Asp), NAA and lutamate (Glu) concentrations with novel J-edited method, based on MEGA-PRESS pulse sequence [1-2]. Thus, main of this study was to evaluate possible causes of NAA reduction in patients with severe TBI (3 month after injury).Methods
In this work 19 children in the age from 9 to 16 years were studied. Patient group consists of 7 (14.3±2.3) children with severe TBI. At the time of the examination, about 3 months (85±16 days) have passed since patients got injured. In the group, there was not any patient in the vegetative state or in the state of akinetic mutism. MR examination was performed under general anesthesia. Control group consists of age-matched volunteers (15.0±1.9) without any cases of TBI or neurological diseases.
All investigations were performed on Phillips Achieva 3.0T scanner. MEGA-PRESS pulse sequence with following parameters (TE/TR=115ms/1900ms, NSA – 8×42, 40ms editing pulses applied at 3.89 ppm and 5.21 ppm) was used for Asp and Glu quantification. Edited spectra were processed by the Amares algorithm in the jMRUi routine. Corresponding PRESS spectra with the same parameters were also performed for obtaining NAA, Cr, Cho and unsuppressed water signal intensities. PRESS spectra were quantified using LC model routine. All Voxels in size of 25×25×30 mm were located in the frontal lobe (normal appearing brain tissue according to MRI examination) (Fig.1.). Absolute concentrations [tNAA], [tCr], [tCho], [Glu], [Asp] were corrected on WM, GM and CSF contaminations, which were quantified using FSL routine. Mann-Whitney U-test was used to reveal intergroup differences. Threshold of significance was set at p<.05.
Results
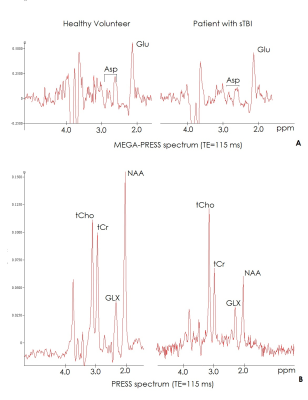
Typical MEGA-PRESS and PRESS spectra are presented on Fig.2. Intergroup analysis revealed significant decrease in [NAA] in the delayed period after trauma (by 65%) as compared to the control (Z=3.51, p <.001, Fig.3). [Asp] is also significantly reduced in the group of patients (Z=3.50, p <.001, Fig.4A) by 61%. There was no significant changes in Glu concentration (Z=1.64, p=n.s, Fig.4A), however, a significant decrease in the [Asp]/[Glu] ratio was found (Z=2.74, p<.05, Fig. 4B). [tCr] showed decreased levels, while [tCho] was increased (Fig.3). Calculated relative contents of GM (0.53 ± 0.04), WM (0.31 ± 0.02) and CSF (0.15 ± 0.03) in the normal group did not significantly differ from the corresponding values (GM (0.51 ± 0.07), WM (0.31 ± 0.05) and CSF 0.18 ± 0.06)) for the patient group. There were no significant correlations between the metabolite concentrations, GM, WM and CSF fractions.Discussion
Decrease in [NAA] may be the inactivation of its synthesis or the activation of hydrolysis. Activation of hydrolysis should lead to the accumulation of Asp. In our study, we observe its fall. In addition, the NAA hydrolysis enzyme, ASPA is inhibited by physiological substrate concentrations, (10-3 mM/ml) [3]. Therefore, it can be assumed that the reason for the decrease in the [NAA] is a disturbance of its synthesis. The simultaneous decrease in the [NAA] and its precursor Asp by 60-65% after severe TBI confirms this assumption.
Glu, main precursor of Asp, comes from the cytosol into the mitochondria through the mitochondrial transporter - glutamate aspartate transporter (Aralar1), which is part of the malate-aspartate shuttle (MAS) [4]. Significant decrease of [Asp] with an unchanged [Glu] found in our study may be a result of the inactivation of the glutamate transport into mitochondria, followed by inhibition of mitochondrial transamination. This conclusion can also be confirmed by the results of study [5], where a significantly reduced synthesis of aspartate is accompanied by decreased consumption of glutamate and malate in mitochondria of knockout mice.
Since stoichiometric Asp-Glu ratio is maintained by the exchange of glutamate with mitochondrial aspartate significant reduction of [Asp]/[Glu] indicates a disruption in the normal functioning of the MAS after sTBI that is in good agreement with previous 13C NMR study of mouse brain extracts [6].
Conclusion
Revealed investigations of metabolites, which are connected by a chain of biochemical transformations (NAA, Asp and Glu), is important and interesting task, that could help more precisely understand causes of changes in metabolite concentrations. In this study for the first time, we revealed potential capabilities of novel J-edited method for simultaneous Asp and Glu quantification [1].Acknowledgements
This work was supported by RFBR Grant 17-04-01149 AReferences
[1] Menshchikov P., Semenova N., Akhadov T. Quantification of cerebral aspartate concentration in vivo using proton magnetic resonance spectroscopy. Bulletin of the Lebedev Physics Institute. 2017;44(3):56-60.
[2] P. Menshchikov, T. Akhadov, N. Semenova. MEGA-PRESS for simultaneous aspartate and glutamate quantification at 3T Magn Reson Mater Phy 2017;27:5499
[3] Kots E, Lushchekina S, Varfolomeev S et al. Role of Protein Dimeric Interface in Allosteric Inhibition of N-Acetyl-Aspartate Hydrolysis by Human Aspartoacylase. J Chem Inf Model. 2017; 57(8):1999-2008.
[4] McKenna M, Waagepetersen H. Schousboe A et al. Neuronal and astrocytic shuttle mechanisms for cytosolic mitochondrial transfer of reducing equivalents: current evidenceand pharmacological tools. Biochem. Pharmacol. 2006;71:399–407.
[5] Jalil M, Begum L, Contreras L et al. Reduced N-acetylaspartate levels in mice lacking aralar, a brain and muscle-type mitochondrial aspartate-glutamate carrier. J Biol Chem 2005; 280:31333–339.
[6] Scafidi S, O’Brein J, Hopkins I et al. Delayed cerebral oxidative glucose metabolism after traumatic brain injury in young rats. J Neurochem. 2009;109(1):189 – 197