0510
Assessing Mild Traumatic Brain Injury (TBI) Induced Optic Neuropathy in Novel Closed-head Injury Mouse Model Using Diffusion Basis Spectrum Imaging (DBSI)1Radiology, Washington University School of Medicine, St Louis, MO, United States, 2Neurology, Washington University School of Medicine, St Louis, MO, United States, 3Hope Center for Neurological Disorders, Washington University School of Medicine, St Louis, MO, United States, 4Biomedical Engineering, Washington Univerisyt is St. Louis, St Louis, MO, United States
Synopsis
Traumatic brain injury (TBI) is a major cause of death and disability worldwide, and mild TBI (mTBI) is the most common type injury. TBI-induced optic neuropathy (ON) has recently raised attention due to subjective complaints of visual impairment. Currently, non-invasive and longitudinal direct assessment in TBI-induced ON is still limited. In the current study, we applied diffusion basis spectrum imaging (DBSI) to a novel surgery-free and closed-head impact model of TBI called modCHIMERA at 3 days post-injury to study TBI-induced ON with histological validation on our imaging metrics.
Introduction
Traumatic brain injury (TBI) is a major cause of death and disability worldwide. The majority of TBI cases are mild or ‘concussive.’ TBI-induced optic neuropathy (ON) has recently raised attention due to subjectively visual complaints and impairment, which is believed to cause by a shock wave to optic canal and lead to acute or chronic optic nerve damage.1, 2 Visual exams, retinal imaging assessment, and histological validation of retina and optic nerve are the primary approaches to study TBI-induced ON. Currently, non-invasive and longitudinal direct assessment in TBI-induced ON is still limited. We previously introduced diffusion basis spectrum imaging (DBSI) to assess mouse optic neuritis3 to reflect co-existing axonal pathologies. In addition, DBSI was also employed to validate corticosteroid treatment efficacy in mouse with optic neuritis and suggested no axonal protection in long-term manner.4 In the current study, we applied DBSI to a novel non-surgical, tunable, monitored TBI model, closed-head impact model of engineered rotational acceleration (CHIMERA) platform, called modCHIMERA (Fig. 1) at 3 days post-injury to study TBI-induced ON with histological validation on our imaging metrics.Materials and Methods
Animal model and ex vivo sample preparation: Five C57BL/6 16-weeks old male mice were underwent experimental traumatic brain injury. Mice were placed on the injury device and a helmet was placed around the head (Fig. 1).5 A 2.1 Joule impact was then delivered to the helmeted head of the animal. The other five mice underwent the same procedure without an impact. At 3 days after injury, mice were perfused and post-fixed in 4% PFA. Tissue was transfer to PBS after 24 hours for ex vivo scan. Ex vivo DBSI protocol: A surface coil was used to cover the whole mouse brain. A 99-direction DBSI was performed on a 4.7-T Agilent small-animal MR scanner utilizing a multiple-echo spin-echo diffusion-weighted sequence.6 All images were obtained with the following acquisition parameters: TR = 3.0 s, TE = 31.5 ms, inter-echo delay = 23.4 ms, Δ = 18 ms, δ = 6 ms, maximal b-value = 3,000 s/mm2, with the same FOV = 15 × 15 mm2, slice thickness = 1 mm, and matrix size = 128 × 128 (before zero-filling). Total scan time was 10 hours and 33 minutes. Data analysis: A lab-developed DBSI computation was performed on DWI data to estimate DBSI-derived axial diffusivity (λǁ), radial diffusivity (λ┴), restricted (putative cellularity) and non-restricted isotropic (putative edema) diffusion tensor fractions. Histology: mice were perfusion fixed immediately after the last in vivo MR measurements for immnunohistochemistry (IHC) staining of SMI-312, MBP, SMI-31, anti-APP and DAPI.Results
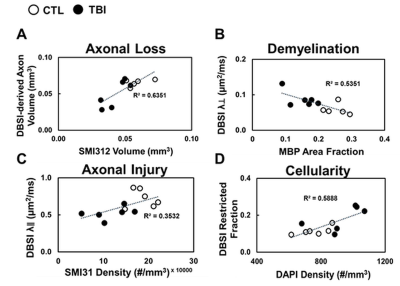
DBSI λǁ and λ┴ were derived from anisotropic diffusion tensor components to reflect axon and myelin integrity, respectively. Comparing to control (CTL) group, reduced DBSI λǁ (Fig. 2 C, p < 0.05) and increased λ┴ (Fig. 2 D, p< 0.05) were observed in TBI optic nerve, suggesting axonal injury and demyelination. DBSI non-restricted and restricted diffusion tensor components were associated with vasogenic edema and cellularity (cell infiltration, cellular activation, severe axonal beading), respectively. Increased DBSI restricted fraction was shown in TBI optic nerves (Fig.2 F, p < 0.05), suggesting increased cellularity. The sensitivity of DBSI non-restricted (putatively edema) might be affected by tissue fixation with 4% PFA. DBSI fiber fraction is the total anisotropic diffusion signal and associated with axonal density (Fig. 3 A). Inflammation and severe injury might lead confounding effects, such as nerve swelling/beading. To assess accurate severity of axonal loss, which is highly associated with irreversible visual impairment, DBSI-derived axon volume (Fig. 3 D) was derived by multiplying fiber fraction (Fig. 3 B) by nerve volume (Fig. 3 C). Reduced DBSI-derived axon volume was shown in some TBI optic nerves (Fig. 3, p < 0.05), suggesting early axonal loss at 3 days post-injury. Compared to CTL optic nerves, the IHC results revealed axonal loss (reduced SMI-312 intensity, Fig. 4 A, F) demyelination (reduced MBP intensity, Fig. 4 B, G), axonal swelling (Fig. 4, white arrows), axonal injury (reduced SMI-31 intensity and increased anti-APP intensity, Fig. 4 C, D, H, I) and increase cellularity (increased DAPI intensity, Fig. 4 E, J). The correlations of DBSI metrics and corresponding IHC markers (Fig.5) suggest that DBSI biomarkers could correctly reflect coexisting pathologies of ON.Conclusions
Our results demonstrate co-existing axonal pathologies in TBI optic nerves at 3 days post injury. The novel modCHIMERA TBI model could be an appropriate model to study TBI-induced ON with controllable severity. Meanwhile, DBSI holds potential to monitor disease progress longitudinally and validate treatment efficacy. Histology of rest tissues is being pursuing for complete sensitivity validation of DBSI biomarkers.Acknowledgements
This work was supported in part by NIH R01-NS047592, P01-NS059560, U01-EY025500, National Multiple Sclerosis Society (NMSS) RG 4549A4/1, RG-1507-05315 and Department of Defense Idea Award W81XWH-12-1-0457.References
1. Tzekov R, Quezada A, Gautier M, et al. Repetitive mild traumatic brain injury causes optic nerve and retinal damage in a mouse model. Journal of neuropathology and experimental neurology 2014;73:345-361. 2. Sarkies N. Traumatic optic neuropathy. Eye (Lond) 2004;18:1122-1125.
3. Lin TH, Chiang CW, Perez-Torres CJ, et al. Diffusion MRI quantifies early axonal loss in the presence of nerve swelling. Journal of neuroinflammation 2017;14:78.
4. Lin TH, Zhan J, Song C, et al. Corticosteroid Treatment Fails to Prevent Long-term Axonal Loss Assessed by Diffusion Basis Spectrum Imaging. 2017;Proc. Intl. Soc. Mag. Reson. Med. 25 (2017):0217.
5. Namjoshi DR, Cheng WH, McInnes KA, et al. Merging pathology with biomechanics using CHIMERA (Closed-Head Impact Model of Engineered Rotational Acceleration): a novel, surgery-free model of traumatic brain injury. Molecular neurodegeneration 2014;9.
6. Tu TW, Budde MD, Xie M, et al. Phase-aligned multiple spin-echo averaging: a simple way to improve signal-to-noise ratio of in vivo mouse spinal cord diffusion tensor image. Magnetic resonance imaging 2014;32:1335-1343.
Figures
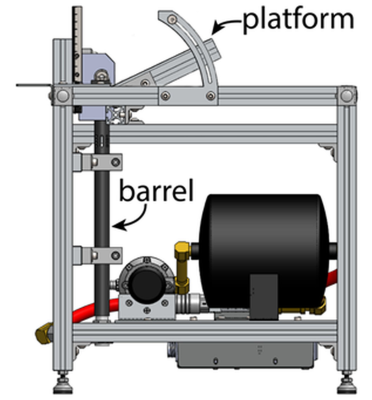
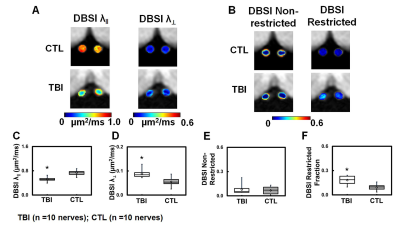
Figure 2 DBSI-derived axial (λǁ) and radial (λ┴) diffusivity maps (A) were derived from anisotropic diffusion tensor components and reflected axonal injury and demyelination, respectively. Significant decreased DBSI λǁ (C) and increased DBSI λ┴ (D) were seen in TBI optic nerves indicated severe axonal injury and demyelination. DBSI-derived restricted (putative cellularity marker) isotropic diffusion tensor fraction maps (B) reflected significant increased cellularity in TBI optic nerves (F).
⋆ indicates p < 0.05
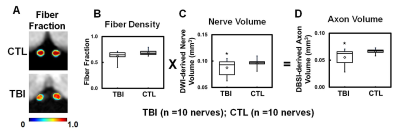
Figure 3 DBSI-derived fiber fraction reflects total signal from anisotropic diffusion tensor components, suggesting axonal fiber density (A). To consider inflammation-induced nerve swelling or atrophy in assessment of axonal loss, DBSI axon volume (D) was derived by fiber fraction (B) and ROI-derived nerve volume (C). Comparing to CTL group, TBI optic nerves showed significant reduced axon volume (D), suggesting axonal loss.
⋆ indicates p < 0.05
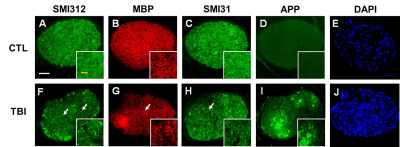
Figure 4 Representative 100x immunohistochemical staining images of total neurofilament (SMI-312, staining both injured and intact axons, A,F), myelin basic protein (MBP, assessing myelin sheaths, B, G), phosphorylated neurofilament (SMI-31, reflecting intact axons, C, H), anti-amyloid precursor protein (APP, axonal injury, D, I) and 4’, 6-dianidino-2-phenylindole (DAPI, detecting nuclei, E, J) from CTL and TBI optic nerves at three days after injury demonstrated TBI optic nerves showed more severe damages. White arrows indicate axonal swelling/beading.
White scale bar: 50 μm
Yellow scale bar: 25 μm