0509
Redundancy and resiliency of arousal mechanisms in traumatic coma patients1Department of Radiology, A.A. Martinos Center for Biomedical Imaging, MGH and Harvard Medical School, Boston, MA, United States, 2Department of Neurology, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, United States, 3Department of Neurology, A. A. Martinos Center for Biomedical Imaging, MGH and Harvard Medical School, Boston, MA, United States
Synopsis
Traumatic brain injury to brainstem/thalamic/hypothalamic/basal-forebrain arousal nuclei is implicated in the pathogenesis of coma. However, due to difficulty in localizing these nuclei with conventional MRI, it is unknown which lesioned nuclei cause coma, and which are compatible with recovery of arousal/consciousness. We mapped our recently developed brainstem arousal-nuclei atlases and current thalamic/hypothalamic/basal-forebrain atlases to 3Tesla susceptibility-weighted-images in twelve acute traumatic-coma patients, who recovered full arousal/consciousness within six-months. We identified multiple combinations of injured brainstem/thalamic arousal-nuclei that cause acute coma, yet are compatible with a full recovery of arousal/consciousness. Thus, there is redundancy and resiliency of arousal mechanisms in traumatic coma.
Introduction
For patients with severe traumatic brain injury (TBI), coma is typically caused1 by disruption of the ascending arousal network that links arousal nuclei in the brainstem, thalamus, hypothalamus and basal forebrain to the cerebral cortex2-4. However, due to the difficulty of localizing these nuclei with conventional MRI, the specific arousal nuclei whose injury is implicated in the pathogenesis of coma have not been identified; moreover, it is unknown which lesioned nuclei are compatible with recovery of arousal/consciousness.Purpose
To identify the constellations of lesioned arousal nuclei that cause coma in patients with acute severe TBI, and that are compatible with the recovery of arousal/consciousness by integrating: (i) our recently developed brainstem nuclei atlases5-7 and current thalamic, hypothalamic and basal forebrain atlases8-9 in standard MNI space with (ii) susceptibility-weighted imaging (SWI) maps of traumatic microbleeds, the hallmark of hemorrhagic traumatic injury.Methods
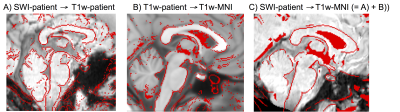
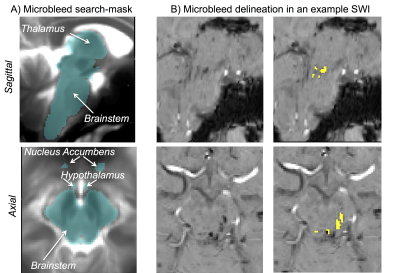
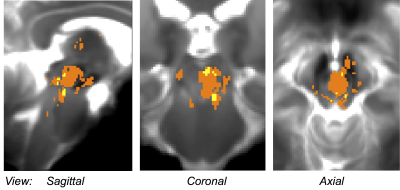
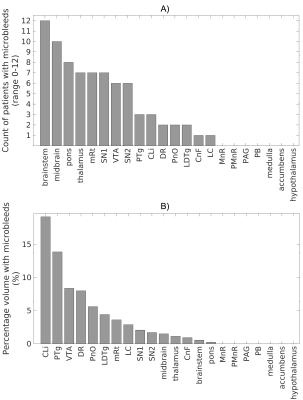
Data acquisition: Sixteen traumatic coma patients (12m/4f, age 29 ± 2) underwent 3 Tesla MRI with IRB approval. Data from two patients were excluded because of motion artifacts. Twelve of the remaining patients recovered full arousal/consciousness by 6 months, as assessed using the Coma Recovery Scale-Revised and Confusional Assessment Protocol, and were included in this study. To identify traumatic microbleed (i.e. lesion) location, a 3D SWI was acquired with spatial-resolution/repetition-time/echo-time/flip-angle/bandwidth/acquisition-time = 0.86mm×0.86mm×1.8mm/30ms/20ms/15o/120Hz/pixel/4’33”. For co-registration purposes, a 3D T1weighted (T1w) multi-echo (ME) MPRAGE was also acquired with spatial resolution/repetition-time/echo-times/inversion-time/flip-angle/GRAPPA factor = 1 mm isotropic/2.53 s/[1.69, 3.55, 5.41, 7.27] ms/1.2 s/7°/3. Data analysis: After bias-field correction, the SWI of each patient was precisely co-registered to the non-linear 6th-generation T1w-MNI152 standard template by concatenating and applying (Advanced-Normalization-Tool10) the following transformations: 1) the 12 degrees-of-freedom affine-transformation (metric: mutual information) computed to co-register the SWI to the T1w-MEMPRAGE; 2) the high-dimensional non-linear transformation (metric: cross correlation) computed to co-register the T1w-MEMPRAGE to the T1w-MNI152. A “traumatic microbleed search mask” was defined as comprising the thalamus (Harvard-Oxford atlas8), hypothalamus (Tailarach atlas9), basal forebrain nucleus accumbens (Harvard-Oxford atlas8), and the brainstem with its 3 subdivisions (pons11, medulla11-12 and midbrain11-12 -for the latter some manual edits were performed by M.B. to cover the rostral midbrain). Within this search-mask, traumatic microbleeds (i.e. SWI hypointensities) were manually delineated on SWIs of each patient in MNI space by a trained microbleed rater (B.E.), using methods developed by our group13. The spatial distribution/overlap of traumatic microbleeds across patients at the voxel level was assessed by computing the sum of microbleeds across patients. Further, we evaluated the spatial distribution/overlap of microbleeds across patients at the nuclei/region level (left and right nuclei/regions were evaluated together). We considered 7 regions (thalamus/hypothalamus/basal forebrain nucleus accumbens/whole brainstem/midbrain/pons/medulla8-9,11-12), 13 brainstem arousal nuclei (periaqueductal gray -PAG4, dorsal-raphe -DR4, median-raphe -MnR4, paramedian-raphe -PMnR5, pontine reticular formation oral part -PnO5, pedunculotegmental nucleus -PTg5, cuneiform nucleus -CnF5, caudal-linear raphe -Cli5, ventral tegmental area -VTA6, mesencephalic reticular formation -mRt6, locus coeruleus -LC6, lateral/medial parabrachial nucleus -PB6, laterodorsal tegmental nucleus -LDTg6), and two subnuclei of the substantia nigra -SN1/2, compatible with pars reticulata/compacta4 (the SN is traditionally considered a midbrain motor nucleus, yet it is a dopaminergic center often associated to the VTA -as the “VTA/SN complex”- and it is involved in the regulation of sleep14-15). Specifically, we counted the number of patients who displayed traumatic microbleeds in each nucleus/region, and the percentage nucleus/region volume affected by the traumatic microbleed.Results
Performance of the SWI-to-MNI coregistration procedure is shown for a representative patient in Figure 1. The traumatic microbleed search mask and an example microbleed delineation in a single patient are shown in Figure 2. The spatial distribution/overlap of microbleeds across patients at the voxel level is shown in Figure 3. Evaluation of the number of patients with nuclei/regions affected by microbleeds and the % nucleus/region volume affected by microbleeds are shown in Figure 4A-B.Discussion
Our findings indicate that there is no single arousal nucleus in the ascending arousal network whose injury is required for the pathogenesis of traumatic coma. Rather, many combinations of injured brainstem and thalamic arousal nuclei can cause acute coma in patients with severe TBI and are compatible with full recovery of arousal/consciousness within 6 months.Conclusions
There is redundancy and resiliency of arousal mechanisms in traumatic coma. The methodology developed here might be applied to improve prognostication and guide the development of therapies for patients with traumatic coma.Acknowledgements
NIH-NIBIB-K01EB019474; NIH-NINDS-K23NS094538; American-Academy-of-Neurology/American-Brain Foundation; James S. McDonnell Foundation. We thank Dr. Salvatore Nigro for providing a mask of the pons in MNI-space.References
1. Rosenblum WI. Immediate, irreversible, posttraumatic coma: a review indicating that bilateral brainstem injury rather than widespread hemispheric damage is essential for its production. J Neuropathol Exp Neurol. 2015;74:198-202.
2. Parvizi J, Damasio A. Consciousness and the brainstem. Cognition. 2001;79:135-159.
3. Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends in Neurosci. 2001;24:726-731.
4. Eban-Rothschild A, Rothschild G, Giardino WJ, et al. VTA dopaminergic neurons regulate ethologically relevant sleep–wake behaviors. Nat Neurosci. 2016;19:1356-1366.
5. Bianciardi M, Toschi N, Edlow BE, et al. Toward an in vivo neuroimaging template of human brainstem nuclei of the ascending arousal, autonomic, and motor systems. Brain Connect. 2015;5:597-607.
6. Bianciardi M, Strong C, Toschi M, et al. A probabilistic template of human mesopontine tegmental nuclei from in vivo 7 T MRI. Neuroimage. 2017; in press.
7. Edlow BL, Takahashi E, Wu O, et al. Neuroanatomic connectivity of the human ascending arousal system critical to consciousness and its disorders. Journal of Neuropathology and Experimental Neurology. 2012;71:531-546.
8. Desikan RS, Ségonne F, Fischl B, et al.. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage, 2006;31:968–980.
9. Lancaster JL, Tordesillas-Gutiérrez D, Martinez M, et al. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp. 2007;28:1194-205.
10. Avants BB, Tustison NJ, Song G, et al. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54:2033–2044.
11. Nigro S, Cerasa A, Zito G, et al. Fully automated segmentation of the pons and midbrain using human T1 MR brain images. PLoS One. 2014;9:e85618.
12. Destrieux C, Fischl B, Dale A, et al. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. 2010;53:1–15.
13. Izzy S, Mazwi NL, Martinez S, et al. Revisiting grade 3 diffuse axonal injury: not all brainstem microbleeds are prognostically equal. Neurocrit Care. 2017; in press.
14. Lima MMS, Andersen ML, Reksidler AB, et al. The role of the substantia nigra pars compacta in regulating sleep patterns in rats. PLoS ONE. 2007;2:e513.
15. Datta S, CurroDossi R, Pare D, et al. Substantia nigra reticulata neurons during sleep-waking states: relation with ponto-geniculo-occipital waves. Brain Res. 1991;566:344-347.
Figures