0508
5-Year Arterial Spin Labeling MR Perfusion sequelae of concussive blast traumatic brain injury1University of Washington Medical Center, Seattle, WA, United States
Synopsis
Concussive blast traumatic brain injury (cbTBI) may benefit from evaluation with advanced MR imaging techniques. Here we report the results of a 5-year follow-up prospective, observational, longitudinal human cohort study evaluating cbTBI using arterial spin labeling, correlated with durable measures of long term outcome taken from an extensive battery of neurobehavioral, neuropsychological, and psychiatric evaluations. Specifically, service members who sustained combat-related cbTBI exhibited significant, regional hypoperfusion 5 years post-injury compared to combat-deployed controls, associated with measures of long term outcome.
PURPOSE
Traumatic brain injury (TBI) comprises a heterogeneous condition with a myriad associated pathoanatomical brain changes. The mild or concussive TBI variant is a challenging diagnosis, in which morphological imaging correlates of clinical symptoms, exam findings, and underlying brain histopathology are only sparsely detected by conventional cross-sectional imaging modalities1-6, rendered inconspicuous by the often subtle aspect of the insult7. An alarming Department of Defense report identified more than 300,000 deployed Iraq and Afghanistan veterans and service members who sustained one or more blast- and/or impact-related TBI event8, 82.3% of which qualified as mild TBI, and up to 15% reporting cognitive and post-concussive symptoms8. Advanced MR neuroimaging techniques, such as arterial spin labeling (ASL), may provide insight and elucidate underlying pathoanatomical brain changes currently not under-appreciated on conventional morphological imaging. The current study sought to assess ASL perfusion abnormalities in veterans and active-duty US military previously exposed to combat-related concussive blast traumatic brain injury (cbTBI).METHODS
Experiment: This 5-year follow-up prospective study with IRB approval and informed consent evaluated two actively deployed military groups: those (subjects) sustaining deployment-related cbTBI exposure plus additional simultaneous head injury (fall, motor vehicle collision, or striking a blunt object), and deployed soldiers (controls) without diagnosis of deployment-related TBI, history of TBI or history of blast exposure. MRI examination performed at 3T (Philips Achieva) included: sequential 3D T1-weighted images (T1) for registration, phase contrast angiography to inform optimal ASL label slab angulation (perpendicular to neck arteries at ~90 mm inferior to the AC-PC line), and a pseudo-continuous ASL (pCASL) preparation using body coil transmission and SENSE 32-channel reception, with the following parameters: TE = 19 ms, TR = 5000 ms, flip angle = 90°, label duration = 1800 ms, post-labeling delay = 2000ms, matrix = 96×96×20, spatial resolution = 3×3×5mm3, 30 control/label pairs and a M0 image with TR = 10000 ms, R = 2.5.
Analysis. All images were motion-corrected and registered to the M0 image. Pairwise subtraction of the pCASL label and control pairs was performed prior to CBF calculation based on ISMRM recommendations9. CBF maps were registered to a standard 2mm MNI template using asl_reg, an ASL-specific FSL utility for optimal standard space image registration. CBF was calculated using the Harvard-Oxford cortical atlas in FSL10, compared between subjects and controls using a 2-sided t-test, and corrected for multiple comparisons using Bonferroni adjustment (p-value for significance = 0.05/48 = 0.001). Clinical evaluation included: structured neurobehavioral interviews, a neuropsychological battery, and structured psychiatric evaluations.
RESULTS
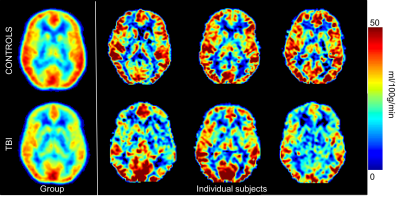
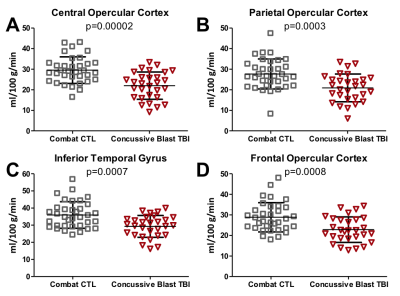
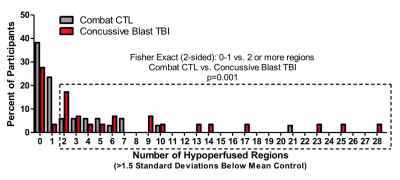
29 individuals (aged 28 ± 6 years, 27 M, years of education = 13 ± 1.5) experienced cbTBI during deployment (between 2008-2013), while 34 individuals (aged 31 ± 8 years, 30 M, years of education = 14 ± 2.3) served as combat-deployed controls (Fig. 1). Cross-sectional analysis identified significant hypoperfusion in 4 of 48 cortical regions of interest (central, parietal, and frontal opercular cortices, and inferior temporal gyrus) in cbTBI subjects compared to controls (Fig. 2). An additional 69% (20/29) of cbTBI subjects had 2 or more regions (Fig. 3) of hypoperfusion (p = 0.001; Fischer’s Exact Test). Logistic regression assessing the association between CBF and clinical outcome data identified a top model that included the diagnosis of TBIDISCUSSION
Our study showed globally decreased hypoperfusion in cbTBI subjects compared to controls, with significant hypoperfusion within the inferior temporal gyrus and central, parietal, and frontal opercular cortices. While most TBI studies focus on traumatic axonal injury within the white matter, few studies have evaluated perfusion deficits in cbTBI. These results are in accordance with prior studies reporting hypoperfusion following cbTBI11,12 and TBI, in general13-15. These results support the role of ASL perfusion imaging in the ongoing evaluation of cbTBI, and combined with imaging measures of anatomical disruptions, may better characterize the pathophysiological sequelae and potential subsequent impairment associated with cbTBI. In this prospective, observational, longitudinal human cohort study evaluating cbTBI using ASL, we report a relationship between regional, chronic hypoperfusion and measures of long term outcome.CONCLUSIONS
Service members who sustained combat-related cbTBI exhibit significant, regional hypoperfusion 5 years post-injury compared to combat-deployed controls, and associated durable measures of long term outcome taken from an extensive battery of neurobehavioral, neuropsychological, and psychiatric evaluations.Acknowledgements
Support for this study was provided by a Department of Defense grant through the Chronic Effects of Neurotrauma Consortium (W81XWH-13-2-0095, C.L. Mac Donald) and by an NIH RO1 grant from NINDS (1R01NS091618-01, C.L. Mac Donald).References
1. Bazarian JJ, Zhong J, Blyth B, Zhu T, Kavcic V, Peterson D. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. J Neurotrauma. 2007;24(9):1447-1459. 2. Inglese M, Makani S, Johnson G, et al. Diffuse axonal injury in mild traumatic brain injury: a diffusion tensor imaging study. Journal of neurosurgery. 2005;103(2):298-303. 3. Scheid R, Preul C, Gruber O, Wiggins C, von Cramon DY. Diffuse axonal injury associated with chronic traumatic brain injury: evidence from T2*-weighted gradient-echo imaging at 3 T. AJNR American journal of neuroradiology. 2003;24(6):1049-1056. 4. FitzGerald DB, Crosson BA. Diffusion weighted imaging and neuropsychological correlates in adults with mild traumatic brain injury. Int J Psychophysiol. 2011;82(1):79-85. 5. Niogi SN, Mukherjee P. Diffusion tensor imaging of mild traumatic brain injury. J Head Trauma Rehabil. 2010;25(4):241-255. 6. Green R, Koshimori Y, Turner G. Research digest. Understanding the organic basis of persistent complaints in mTBI: findings from functional and structural neuroimaging. Neuropsychol Rehabil. 2010;20(3):471-478. 7. Lee B, Newberg A. Neuroimaging in traumatic brain imaging. NeuroRx. 2005;2(2):372-383. 8. DoD worldwide numbers for TBI. Department of Defense; 2012. dvbic.dcoe.mil/dod-worldwide-numbers-tbi. Accessed October 17, 2017. 9. Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2014. 10. Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. NeuroImage. 2012;62(2):782-790. 11. DeWitt DS, Prough DS. Blast-induced brain injury and posttraumatic hypotension and hypoxemia. J Neurotrauma. 2009;26(6):877-887. 12. Clark AL, Bangen KJ, Sorg SF, et al. Dynamic association between perfusion and white matter integrity across time since injury in Veterans with history of TBI. Neuroimage Clin. 2017;14:308-315. 13. Wang Y, West JD, Bailey JN, et al. Decreased cerebral blood flow in chronic pediatric mild TBI: an MRI perfusion study. Dev Neuropsychol. 2015;40(1):40-44. 14. Amen DG, Willeumier K, Omalu B, Newberg A, Raghavendra C, Raji CA. Perfusion Neuroimaging Abnormalities Alone Distinguish National Football League Players from a Healthy Population. J Alzheimers Dis. 2016;53(1):237-241. 15. Barlow KM, Marcil LD, Dewey D, et al. Cerebral Perfusion Changes in Post-Concussion Syndrome: A Prospective Controlled Cohort Study. J Neurotrauma. 2017;34(5):996-1004.Figures