0507
The Evolution of White Matter Microstructural Changes after Mild Traumatic Brain Injury: A Longitudinal DTI and NODDI Study1Radiology & Biomedical Imaging, University of California San Francisco, San Francisco, CA, United States, 2Department of Radiology, University of Washington, Seattle, WA, United States, 3Brain and Spinal Cord Injury Center, San Francisco General Hospital and Trauma Center, San Francisco, CA, United States, 4Department of Neurological Surgery and Brain and Spinal Injury Center, University of California San Francisco, San Francisco, CA, United States, 5Department of Bioengineering & Therapeutic Sciences, University of California, San Francisco, San Francisco, CA, United States
Synopsis
Problem: Mild traumatic brain injury (mTBI) can result in long-term sequelae. Lack of sensitive biomarkers makes diagnosis challenging. Methods: Cross-sectional and longitudinal study of 40 mTBI patients at 2 weeks and 6 months after injury. Diffusion tensor imaging and multishell neurite orientation dispersion and density imaging (NODDI) parameters were assessed. Results: Cross-sectional analysis between patients at 2-weeks and controls revealed a decrease of fractional anisotropy and increase of mean diffusivity in the patient group together with elevated free water values. Longitudinally, after mTBI, a decline in neurite density was observed. Conclusions: NODDI measurements are sensitive imaging biomarkers for the subtle underlying white matter pathology after mTBI.
INTRODUCTION
Long term structural brain changes and cognitive sequelae after mild traumatic brain injury (mTBI) are understudied. The lack of sensitive imaging biomarkers to detect subtle brain injury challenges the diagnosis and treatment of mTBI1. Diffusion tensor imaging (DTI) is the most widely used technique worldwide to study the microstructural properties of white matter in vivo2 despite some known limitations such as the assumption of Gaussian diffusion3. Neurite orientation dispersion and density imaging (NODDI), using multishell diffusion MRI, has been proposed to capture additional information about the biophysical microarchitecture of white mater4. Specifically, three measurements are calculated: a marker of neurite density index (NDI); orientation dispersion index (ODI), and volume fraction of the isotropic compartment (FISO) which represents free water. Comparing DTI to NODDI serially after mTBI, we hypothesize that the early microstructural white matter changes of mTBI are driven by increases of free water, such as from neuroinflammation, whereas longer-term changes reflect decreases of neurite density due to evolving white matter degeneration.METHODS
A cohort of 40 mTBI patients were enrolled at the San Francisco General Hospital as part of the prospective multi center project Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI)5. Patients were included within 24h after injury upon meeting the American Congress of Rehabilitation Medicine (ACRM) criteria for mTBI6. Other inclusion criteria included: age 18-55yo, acute brain CT as part of clinical care within the 24h of injury, and no previous psychiatric or neurological disorder. Imaging acquisitions were acquired at 2 weeks and 6 months after injury. Images were acquired in a 3T GE MR750 scanner equipped with an eight channel phased array head radiofrequency coil (GE Healthcare, Waukesha, WI). Whole-brain DTI was performed with a multi-slice single-shot spin echo echoplanar pulse sequence (echo time [TE] = 81 ms; repetition time [TR] = 9 sec) using 60 diffusion-encoding directions, isotropically distributed over the surface of a sphere with electrostatic repulsion, acquired at b = 1300 sec/mm2 and b = 3000 sec/mm2, eight acquisitions at b = 0 sec/mm2, slices of 2.7-mm thickness each with no gap between slices, a 128 x128 matrix, and a field of view (FOV) of 350 x 350 mm. Fourteen control participants were matched to a subsample of patients (n=25) to be compared cross-sectionally in the acute stage at 2 weeks post-injury with the same scanner and parameters of acquisition. FSL tools were used to perform motion correction, brain extraction, and DTIfit for DTI maps calculation7,8. NODDI metrics were derived using the NODDI toolbox v0.9 (http://www.nitrc.org/projects/noddi_toolbox). Mean fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), radial diffusivity (RD), NDI, ODI, and FISO values were obtained from each scan's FA skeleton map by using Tract-Based Spatial Statistics (TBSS) pipeline. We used non-parametric permutation testing voxel-wise analysis for group comparison among patients and controls9.RESULTS
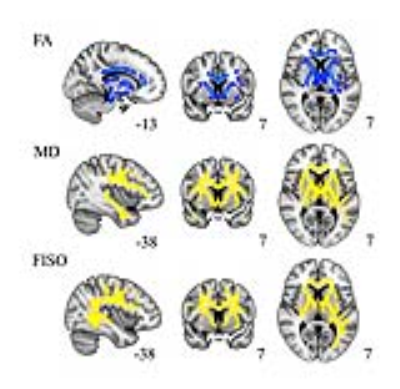
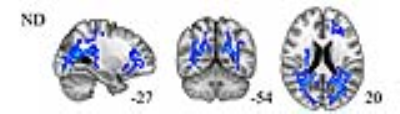
Cross-sectional comparison at 2 weeks, patients and controls: patients showed decreased FA in the body of the corpus callosum, anterior limb of internal capsule, anterior corona radiata, external capsule and cingulum, and increases in MD and FISO additionally in the genu and splenium of the corpus callosum, posterior limb of internal capsule, superior longitudinal fasciculi and fronto-occipital tracts. See Figure1. Longitudinal comparison, patients at 2weeks vs 6months: patients showed decreases over time in NDI mainly in posterior brain tracts described and displayed in Figure2. No significant changes in DTI metrics were observed.CONCLUSIONS
NODDI measurements are sensitive imaging biomarkers for the subtle underlying white matter pathology after mTBI. Our results show that the early decrease of FA and increase of MD after mTBI, which are primarily anterior in the brain, correspond to white matter regions of elevated FISO, possibly reflecting neuroinflammation. The longer-term changes from 2 weeks to 6 months after mTBI are marked by declining neurite density in predominantly posterior white matter, suggesting neurodegeneration for which NODDI appears more sensitive than any DTI metrics such as FA. Future research studies will explore the prognostic significance of these white matter microstructural changes for cognition and behavior after mTBI.Acknowledgements
TRACK-TBI InvestigatorsReferences
- Levin HS, Diaz-Arrastia RR. Diagnosis, prognosis, and clinical management of mild traumatic brain injury. Lancet Neurol. 2014;14(5):506-17.
- Basser PJ, Mattiello J, Bihan DL. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66(1):259-267.
- Jones DK, Cercignani M. Twenty-five pitfalls in the analysis of diffusion MRI data. NMR Biomed. 2010;23(7):803-820.
- Zhang H, Hubbard PL, Parker GJM, Alexander, D. Axon diameter mapping in the presence of orientation dispersion with diffusion MRI. Neuroimage 2011;56(3):1310-1315.
- Yuh EL, Cooper SR, Mukherjee P, et al. Diffusion tensor imaging for outcome prediction in mild traumatic brain injury: a TRACK-TBI study. J Neurotrauma 2014;31(17):1457-77.
- American Congress of Rehabilitation Medicine. Definition of mild traumatic brain injury. J Head Trauma Rehabil. 1993;8(3):86-87.
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143-55.
- Jenkinson M., Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002;17(2):825-41.
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15(1):1-25.
Figures