0506
Ribbon Tractography Reveals Reorientation of White Matter in the Corpus Callosum Following Severe Traumatic Brain Injury1Harvard Medical School - Massachusett General Hospital - Martinos Center for Biomedical Imaging, Boston, MA, United States, 2Department of Neurology, Massachusetts General Hospital, Boston, MA, United States, 3Rutgers Robert Wood Johnson Medical School, New Brunswick, NJ, United States, 4University of São Paulo, São Paulo, Brazil
Synopsis
Traditional streamline tractography does not capture the changes in fiber twisting resulting from traumatic brain injury. We used ribbon tractography to quantify the acute directional changes in white matter tracts in patients with traumatic brain injury. We identified significant changes in the corpus callosum of these patients both as compared to healthy controls and between the acute and follow-up scans of a subset of patients. These findings reveal alterations in the architecture of the corpus callosum within and beyond the area of injury and offer a potential marker for structural changes that are not detected by standard techniques.
Introduction
Traumatic axonal injury (TAI) is considered the most devastating consequence of traumatic brain injury.1 The biomechanical basis of TAI is rotational acceleration-deceleration resulting in shear strain deformation and subsequent disconnection of axons.2 Diffusion tensor imaging (DTI) detects TAI with greater sensitivity than conventional MRI.3 However, streamline tractography only considers the principal fiber direction and does not account for acute directional changes in white matter (WM) such as twisting. Ribbon tractography quantifies the acute directional changes in WM tracts that are not captured by streamline tractography.4 We hypothesize that changes in WM twisting angle (TA) caused by TAI lesions provide insight into the deformation of axons following trauma that complement changes in other DTI metrics. Here, we use ribbon tractography to quantify the TAs of WM in the corpus callosum (CC) of 11 patients with severe traumatic brain injury and compare the evolution of these metrics in a subset of patients who were imaged 6-12 months post-injury.Methods
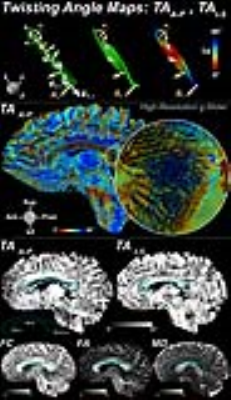
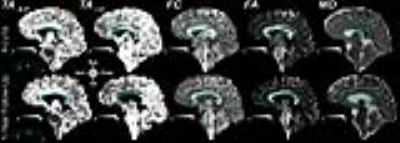
Eleven patients (27.9±9.2years) with severe traumatic brain injury (admission Glasgow Coma Scale scores of 3-8) were enrolled under an IRB-approved protocol with surrogate consent. Patients underwent an acute DTI scan (≤7days) and had TAI lesions involving the body and splenium of the CC, with two having lesions involving the genu. Four underwent a follow-up DTI scan at 6-12 months post-injury. Scans were performed on a 3T MRI scanner (Siemens) in the Neurosciences ICU at Massachusetts General Hospital. DTI were acquired in the whole brain with a spin-echo EPI sequence (TE/TR=97/6800ms) at 2mm isotropic resolution (GRAPPA=2) using 60 diffusion-encoding directions at b=0 and 2000s/mm2. Six healthy control (HC) subjects were scanned on the 3T Connectome scanner (Siemens) with a 300mT/m gradient array5 using the gSlider-SMS technique6 (gSliderxMB factor=5x2) at 750µm isotropic resolution; TE/TReff=80/2200ms; 2 repetitions of 128 directions at b=0 and 1500s/mm2. Ribbon tracking was performed via numerical integration of the primary eigenvector field,4 with ribbon width and thickness proportional to the secondary and tertiary eigenvectors, respectively. TA were defined at each ribbon point as the angles between the local normal vector of the ribbon and the Right-Left (TAR-L), Anterior-Posterior (TAA-P), and Inferior-Superior (TAI-S) global frame axes. Mean diffusivity (MD), fractional anisotropy (FA) and fiber directional coherence (FC) were also computed.7 Semi-automated segmentation delineated the CC and identified boundaries of the genu, body, and splenium. WM twisting was quantified by region (genu, body, and splenium). Group comparisons were performed using the Wilcoxon rank-sum test and Tukey’s analysis.Results
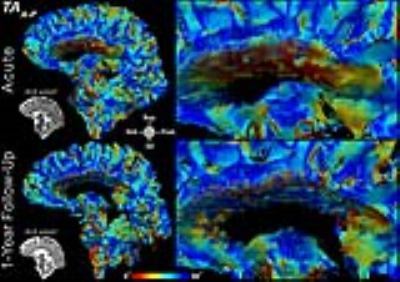
Figures 1 and 2 show results from ribbon tracking in the CC of a healthy control and from the acute and one-year follow-up scans of a patient with a lesion in the body of the CC. Marked increase in TAA-P and decrease in TAI-S was seen in the body of the CC between the acute and follow-up scans that accompanies a decrease in FA and increase in MD. Ribbon tracking through the body of the CC in the same patient highlights the change in TA and relative thinning of the body of the CC at 1-year follow-up (Figure 3). Figure 4 shows the mean±SD TA, FC, FA, and MD in the body of the CC for all patients at the time of the acute scan as compared to the HCs. A significant decrease in TAA-P (p=0.015) and increase in TAI-S (p=0.003) was seen in the body of the CC in patients as compared to HCs. A significant decrease in FC (p=0.0009) and increase in MD (p=0.015), as well as a trend toward decrease in FA (p=0.05) were observed in this region. Significant differences in FA, FC, and MD were seen between patients and HCs in the genu and splenium, but no significant group-level differences in TAA-P or TAI-S were observed in these regions. In the subset of patients with follow-up imaging, at least one of the TAs showed significant differences between the acute and follow-up scans throughout the CC.Discussion
We found widespread longitudinal alterations in TA reflecting reorientation of fibers in the CC following traumatic brain injury. Changes in TA were most pronounced between patients and HCs in the body of the CC. In the subset of patients with follow-up scans, longitudinal changes in TA were also observed in all regions of the CC. These findings suggest that TAI may fundamentally alter the structure of the CC beyond the area of injury, which is not detected by streamline tractography. Elucidating the clinical implications of WM twisting in the acute and chronic setting following trauma will require further study in a larger group of patients with longitudinal imaging.Acknowledgements
No acknowledgement found.References
1. Gentry LR, Godersky JC, Thompson B. MR imaging of head trauma: review of the distribution and radiopathologic features of traumatic lesions. AJR American journal of roentgenology. 1988;150(3):663-672.
2. Adams JH, Jennett B, Murray LS, Teasdale GM, Gennarelli TA, Graham DI. Neuropathological findings in disabled survivors of a head injury. Journal of neurotrauma. 2011;28(5):701-709.
3. Edlow BL, Copen WA, Izzy S, et al. Diffusion tensor imaging in acute-to-subacute traumatic brain injury: a longitudinal analysis. BMC Neurology. 2016;16:2.
4. Mekkaoui C, Jackowski MP, Setsompop K, et al. Characterization of white matter tortuosity using high-resolution gSlider SMS diffusion imaging. Paper presented at: International Society for Magnetic Resonance in Medicine2017; Honolulu, Hawaii.
5. Setsompop K, Kimmlingen R, Eberlein E, et al. Pushing the limits of in vivo diffusion MRI for the Human Connectome Project. NeuroImage. 2013;80:220-233.
6. Setsompop K, Fan Q, Stockmann J, et al. High-resolution in vivo diffusion imaging of the human brain with generalized slice dithered enhanced resolution: Simultaneous multislice (gSlider-SMS). Magn Reson Med. 2017 Mar 5. doi: 10.1002/mrm.26653.
7. Klingberg T, Vaidya CJ, Gabrieli JD, et al. Myelination and organization of the frontal white matter in children: a diffusion tensor MRI study. Neuroreport. 1999 Sep 9;10(13):2817-21.
Figures