0503
Multi-parametric MRI data fusion reveals acute and persistent brain changes in concussed female varsity rugby playersKathryn Y. Manning1,2, Alberto Llera3, Robert Bartha1,2, Gregory A. Dekaban4,5, Christy Barreira4, Arthur Brown 4,6, Lisa Fischer7, Tatiana Jevreomvic7, Kevin Blackney4, Timothy J. Doherty8, Douglas D. Fraser9, Jeff Holmes10, Christian F. Beckmann3,11,12, and Ravi S. Menon1,2
1Medical Biophysics, Western University, London, ON, Canada, 2Centre for Functional and Metabolic Mapping, Robarts Research Institute, London, ON, Canada, 3Donders Centre for Cognitive Neuroimaging, Radboud University, Nijmegen, Netherlands, 4Molecular Medicine, Robarts Research Institute, London, ON, Canada, 5Microbiology and Immunology, Western University, London, ON, Canada, 6Anatomy and Cell Biology, Western University, London, ON, Canada, 7Primary Care Sport Medicine, Fowler Kennedy Sport Medicine, London, ON, Canada, 8Physical Medicine and Rehabilitation, Western University, London, ON, Canada, 9Paediatrics Critical Care Medicine, London Health Sciences Centre, London, ON, Canada, 10Occupational Therapy, Western University, London, ON, Canada, 11Cognitive Neuroscience, Radboud University Medical Centre, Nijmegen, Netherlands, 12Centre for Functional MRI of the Brain (FMRIB), Oxford University, Oxford, United Kingdom
Synopsis
Multi-parametric 3T MRI data was acquired in female varsity rugby players during the in- and off-season and compared to concussed teammates acutely (24-72 hours) and longitudinally (3- and 6-months) after a mild traumatic brain injury (mTBI). Using linked independent component analysis, we found acute and prolonged resting state functional connectivity and diffusion white matter microstructure changes that persisted beyond symptomatic recovery and clearance to return to play. These fused components also significantly correlated with aspects of concussion history and were robust and consistent across model orders and within individual concussed athletes longitudinally.
Introduction
Concussion or mild traumatic brain injury (mTBI) remains a critical and poorly understood health issue, especially for young athletes participating in contact sports. While MRI studies have reported variable changes post-concussion, longitudinal multi-modal studies are required to gain a more complete understanding of the brain’s response and the risk of further concussions or more serious neurological disorders like second impact syndrome and chronic traumatic encephalopathy (CTE)1,2. With increasing study design complexities, comparing different MRI measures becomes non-trivial. Linked independent component analysis (LICA) is a data-driven approach that can elegantly fuse different modalities to understand biologically relevant modes of shared inter-subject variance3. Here we examine female varsity rugby players using multi-parametric MRI longitudinally after a diagnosed concussion and compare to their non-concussed team mates over a period of 5 years to examine (a) acute and (b) persistent brain alterations after a concussion, and (c) the relationship of any changes to subject-specific clinical measures or concussion history.Methods
Diffusion tensor imaging (DTI) with 64 gradient directions and a 10-minute resting state functional MRI (RS-fMRI) scan were acquired on a 3T MRI (Siemens) in non-concussed rugby players throughout the in- and off-season and compared to concussed teammates (n = 21) acutely (24-72 hours) and longitudinally (3- and 6-months) after a diagnosed mTBI. Turbo spin echo high-resolution anatomical images were used to confirm no hematological pathology indicative of more serious TBI. Diffusion data was eddy current corrected and analyzed using non-linearly registered tract-based spatial statistics to create FA-skeletonized maps (Figure 1a) of fractional anisotropy (FA), mean (MD) and axial (AD) diffusion4. RS-fMRI data was preprocessed in FSL and denoised using single-subject ICA5. Group ICA and dual regression algorithms were applied to temporally concatenated cleaned data to identify average resting state networks (RSN) and create subject-specific RSN maps, respectively, including the default mode network (DMN), cerebellar network, and the executive network (Figure 1b)6. DTI and RS-fMRI maps were concatenated by subject and analyzed using LICA with a modest model order (i = 15) given the longitudinal nature of this dataset. Components related to the subject’s age at the time of the scan or dominated by a single-subject (>10% variance) were not considered. The analysis was repeated for +/- 1 model orders to confirm robustness of relevant components that had associated subject weightings with a significant main effect for group using an AVONA with Tukey post-hoc correction or significantly correlated with clinical measures (Sports Concussion Assessment Tool [SCAT] scores) or concussion history (Bonferroni corrected, p < 0.05).Results
We identified two components that had a significant main effect for group (p < 0.001). Component 4 (Figure 2) was heavily influenced by diffusion metrics (Figure 1c) and off-season subject weights were significantly higher than in-season (p < 0.05) or acute post-concussion data (p < 0.001), generally suggesting decreased MD, AD within the corpus callosum, inferior deep white matter structures and corticospinal tracts after concussion and possibly even sub-concussive impacts (Figure 3a). Component 5 had contributions from all 6 MRI metrics (Figure 4) and had significantly higher subject weights for 3- (p < 0.005) and 6-months (p < 0.0005) post-concussion compared to in- and off-season data (Figure 5a). Furthermore, when looking at only non-concussed athletes, we observed significantly higher component weights for subjects with any concussion history compared to those without (p < 0.05) that also correlated significantly with the total number of concussions (r = 0.51, p < 0.01 x 10-10). This indicated decreases in MD and AD within the body and splenium of the corpus callosum, fornix and thalamus with some spatially related changes in functional connectivity beyond symptomatic recovery after mTBI. We investigated component 4 and 5 subject weightings longitudinally in concussed athletes using repeated measures ANOVA (n = 9) and found consistent changes in each individual player (Figure 3b and 4b).Discussion
In this longitudinal multi-parametric MRI study of concussed and non-concussed female rugby players we identified linked components that described both acute and persistent brain changes after a concussion that also related to concussion history. We also found significant effects between in- and off-season data in the absence of an mTBI that warrants further investigation in order to isolate the effects of subconcussive impacts on the brain. These data-driven techniques were able to fuse functional connectivity and diffusion microstructure changes. Significant components were robust across model orders and within individual subjects longitudinally. MRI changes persisted beyond symptomatic recovery and a physician’s clearance to return to play. The increased risks of multiple concussions or second impact syndrome remain to be elucidated, and future research efforts need to follow athletes further to understand how or if these longitudinal changes recover or manifest into neurodegenerative processes.Acknowledgements
The authors have no conflicts of interest to disclose and would like to thank and commend the players and their parents/guardians for their willingness to participate in this study. We would like to acknowledge the excellent support provided by the coaches and MRI technicians.References
1. Bigler ED, Maxwell WL. Neuropathology of mild traumatic brain injury: Relationship to neuroimaging findings. Brain Imaging Behav. 2012; 6:108–136. 2. Daneshvar DH, Riley DO, Nowinski CJ, et al. Long-term consequences: effects on normal development profile after concussion. Phys Med Rehabil Clin N Am. 2011; 22: 683–700. 3. Groves AR, Beckmann CF, Smith SM, et al. Linked independent component analysis for multimodal data fusion. Neuroimage. 2011; 54: 2198–2217. 4. Smith SM, Jenkinson M, Johansen-Berg H. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006; 31:1487-1505. 5. Griffanti L, Douaud G, Bijsterbosch J, et al. Hand classification of fMRI ICA noise components. Neuroimage. 2017; 154: 188–205. 6. Smith SM, Fox PT, Miller KL, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc. Natl. Acad. Sci. 2009; 106: 13040–13045.Figures
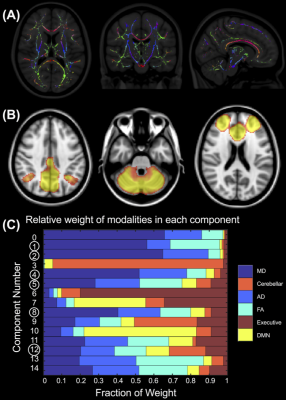
(A) Skeletonized FA map coloured by primary diffusion direction. (B) Group ICA resting state networks scaled by p-value [0, 0.001]. From left to right, the DMN, cerebellar and executive network. (C) The relative contribution of each modality or MRI metric to each LICA component. Circled components indicate relevant components that were significantly correlated with clinical measures, concussion history or that had a significant main effect for group.
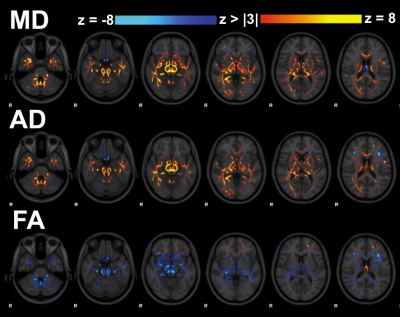
LICA
component 4 that was dominated by diffusion metrics. Yellow/red display positive weightings and blue show negative weightings associated with this component in inferiorly located white matter tracts near the brain stem as well as the cingulum, corpus callosum, and corticospinal tracts.
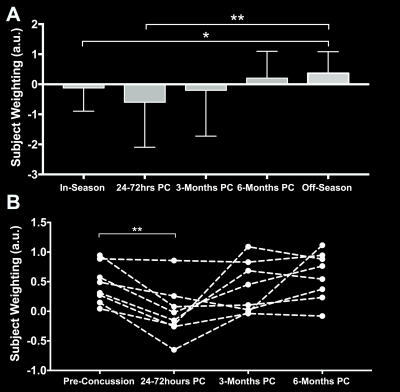
Component
4 results. (A) Average subject weights per group with significant differences shown through corrected Tukey post-hoc tests and (B) longitudinal data in
concussed subjects with complete datasets with significant repeated measures
ANOVA differences shown using star symbols (*p < 0.05, **p < 0.01)
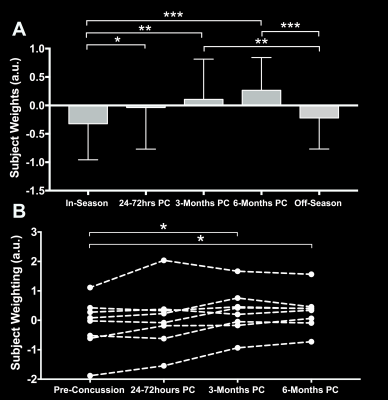
Component
5 results. (A) Average subject weights per group with significant difference
shown through corrected Tukey post-hoc tests and (B) longitudinal data in
concussed subjects with complete datasets with significant repeated measures
ANOVA differences shown using star symbols (*p < 0.05, **p < 0.01, ***p <
0.001)
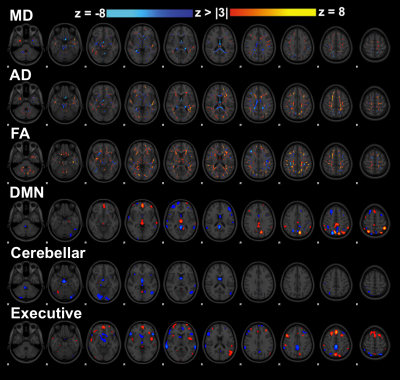
LICA
component 5 with contributions from all 6 MRI metrics. Yellow/red display positive weightings and blue show negative weightings associated with this component. Diffusion metrics along the cingulum, corpus callosum, and fornix dominate, along with spatially related associations with several functional connectivity networks.